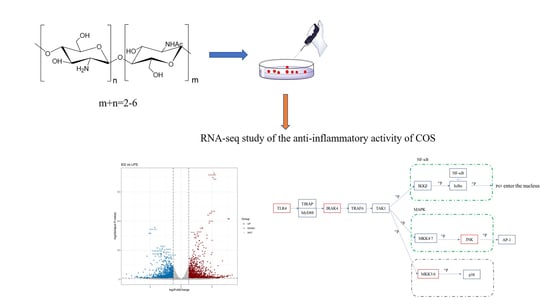
Pleiotropic Modulation of Chitooligosaccharides on Inflammatory Signaling in LPS-Induced Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
2.3. RNA-seq and Data Analysis
2.4. Real-Time qPCR (RT-qPCR) and Western Blotting (WB)
2.5. Statistical Analysis
3. Results
3.1. RNA-seq Data-Quality Assessment
3.2. Differentially Expressed Genes (DEGs) Analysis
3.3. RT-qPCR
3.4. Functional Annotation and Enrichment Analysis of DEGs
3.5. Pathways Validation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Kang, K.H.; Je, J.Y.; Pham, H.N.D.; Byun, H.G.; Kim, S.K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1465. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y.I. Effects of the molecular weight and the degree of deacetylation of chitosan oligosaccharides on antitumor activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Sanchez, A.; Mengibar, M.; Rivera-Rodriguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Chatelain, P.G.; Pintado, M.E.; Vasconcelos, M.W. Evaluation of chitooligosaccharide application on mineral accumulation and plant growth in Phaseolus vulgaris. Plant Sci. 2014, 215–216, 134–140. [Google Scholar] [CrossRef]
- Djordjevic, M.A.; Bezos, A.; Susanti; Marmuse, L.; Driguez, H.; Samain, E.; Vauzeilles, B.; Beau, J.M.; Kordbacheh, F.; Rolfe, B.G.; et al. Lipo-chitin oligosaccharides, plant symbiosis signalling molecules that modulate mammalian angiogenesis in vitro. PLoS ONE 2014, 9, e112635. [Google Scholar] [CrossRef]
- Barman, P.K.; Mukherjee, R.; Prusty, B.K.; Suklabaidya, S.; Senapati, S.; Ravindran, B. Chitohexaose protects against acetaminophen-induced hepatotoxicity in mice. Cell Death Dis. 2016, 7, e2224. [Google Scholar] [CrossRef] [Green Version]
- Jing, B.; Cheng, G.; Li, J.; Wang, Z.A.; Du, Y. Inhibition of Liver Tumor Cell Metastasis by Partially Acetylated Chitosan Oligosaccharide on A Tumor-Vessel Microsystem. Mar. Drugs 2019, 17, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.S.; Taguchi, T.; Sakai, K.; Matahira, Y.; Wang, M.W.; Miwa, I. Effect of chitobiose and chitotriose on carbon tetrachloride-induced acute hepatotoxicity in rats. Biol. Pharm. Bull. 2005, 28, 1971–1973. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Liao, M.; Zhu, Y.; Hu, X.; Wang, J. The protective role of Chitooligosaccharides against chronic ulcerative colitis induced by dextran sulfate sodium in mice. J. Funct. Foods 2021, 87, 104809. [Google Scholar] [CrossRef]
- Gu, M.; Pan, S.; Li, Q.; Qi, Z.; Deng, W.; Bai, N. Chitosan and chitooligosaccharides attenuate soyabean meal-induced intestinal inflammation of turbot (Scophthalmus maximus): Possible involvement of NF-small ka, CyrillicB, activator protein-1 and mitogen-activated protein kinases pathways. Br. J. Nutr. 2021, 126, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, A.; Wang, H.; Su, J.; Liu, X. Mannose Receptor Mediates the Activation of Chitooligosaccharides on Blunt Snout Bream (Megalobrama amblycephala) Macrophages. Front. Immunol. 2021, 12, 686846. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fang, B.; Yong, Y.; Li, X.; Gong, D.; Li, J.; Yu, T.; Gooneratne, R.; Gao, Z.; Li, S.; et al. Chitosan oligosaccharide-mediated attenuation of LPS-induced inflammation in IPEC-J2 cells is related to the TLR4/NF-kappaB signaling pathway. Carbohydr. Polym. 2019, 219, 269–279. [Google Scholar] [CrossRef]
- Zhao, Q.; Yin, L.; Zhang, L.; Jiang, D.; Liu, L.; Ji, H. Chitoheptaose Promotes Heart Rehabilitation in a Rat Myocarditis Model by Improving Antioxidant, Anti-Inflammatory, and Antiapoptotic Properties. Oxid. Med. Cell. Longev. 2020, 2020, 2394704. [Google Scholar] [CrossRef] [Green Version]
- Jitprasertwong, P.; Khamphio, M.; Petsrichuang, P.; Eijsink, V.G.H.; Poolsri, W.; Muanprasat, C.; Rangnoi, K.; Yamabhai, M. Anti-inflammatory activity of soluble chito-oligosaccharides (CHOS) on VitD3-induced human THP-1 monocytes. PLoS ONE 2021, 16, e0246381. [Google Scholar] [CrossRef]
- Das, P.; Panda, S.K.; Agarwal, B.; Behera, S.; Ali, S.M.; Pulse, M.E.; Solomkin, J.S.; Opal, S.M.; Bhandari, V.; Acharya, S. Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis. Sci. Rep. 2019, 9, 2904. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.C.; Spindola, H.; de Sousa, V.; Santos-Silva, A.; Pintado, M.E.; Malcata, F.X.; Carvalho, J.E. Anti-inflammatory activity of chitooligosaccharides in vivo. Mar. Drugs 2010, 8, 1763–1768. [Google Scholar] [CrossRef]
- Ma, P.; Liu, H.-T.; Wei, P.; Xu, Q.-S.; Bai, X.-F.; Du, Y.-G.; Yu, C. Chitosan oligosaccharides inhibit LPS-induced over-expression of IL-6 and TNF-α in RAW264.7 macrophage cells through blockade of mitogen-activated protein kinase (MAPK) and PI3K/Akt signaling pathways. Carbohydr. Polym. 2011, 84, 1391–1398. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xu, Q.S.; Du, Y.G.; Xu, J. Chitosan oligosaccharides block LPS-induced O-GlcNAcylation of NF-kappaB and endothelial inflammatory response. Carbohydr. Polym. 2014, 99, 568–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Liu, M.; Liu, Q.; Wang, W.; Du, Y.; Yin, H. The inhibition of LPS-induced inflammation in RAW264.7 macrophages via the PI3K/Akt pathway by highly N-acetylated chitooligosaccharide. Carbohydr. Polym. 2017, 174, 1138–1143. [Google Scholar] [CrossRef]
- Deng, J.J.; Li, Z.Q.; Mo, Z.Q.; Xu, S.; Mao, H.H.; Shi, D.; Li, Z.W.; Dan, X.M.; Luo, X.C. Immunomodulatory Effects of N-Acetyl Chitooligosaccharides on RAW264.7 Macrophages. Mar. Drugs 2020, 18, 421. [Google Scholar] [CrossRef] [PubMed]
- Morin, R.; Bainbridge, M.; Fejes, A.; Hirst, M.; Krzywinski, M.; Pugh, T.; McDonald, H.; Varhol, R.; Jones, S.; Marra, M. Profiling the HeLa S3 transcriptome using randomly primed cDNA and massively parallel short-read sequencing. BioTechniques 2008, 45, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Nagalakshmi, U.; Wang, Z.; Waern, K.; Shou, C.; Raha, D.; Gerstein, M.; Snyder, M. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 2008, 320, 1344–1349. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Hao, W.T.; Li, K.C.; Ge, X.Y.; Yang, H.Y.; Xu, C.J.; Liu, S.; Yu, H.H.; Li, P.C.; Xing, R.G. The Effect of N-Acetylation on the Anti-Inflammatory Activity of Chitooligosaccharides and Its Potential for Relieving Endotoxemia. Int. J. Mol. Sci. 2022, 23, 8205. [Google Scholar] [CrossRef]
- Sun, J.; Sun, J.; Song, B.; Zhang, L.; Shao, Q.; Liu, Y.; Yuan, D.; Zhang, Y.; Qu, X. Fucoidan inhibits CCL22 production through NF-kappaB pathway in M2 macrophages: A potential therapeutic strategy for cancer. Sci. Rep. 2016, 6, 35855. [Google Scholar] [CrossRef]
- Allan, R. Vaccinia tricks Toll. Genome Biol. 2000, 1, reports0079. [Google Scholar] [CrossRef]
- Chang, L.; Karin, M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 40. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhao, L.H.; Yu, Q.Q. Receptor-mediated stimulatory effect of oligochitosan in macrophages. Biochem. Biophys. Res. Commun. 2004, 317, 414–420. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Y.; Ling, T.; Zhao, C.; Li, Y.; Geng, M.; Gai, S.; Qi, W.; Luo, X.; Chen, L.; et al. Chitosan Oligosaccharides Alleviate Colitis by Regulating Intestinal Microbiota and PPARgamma/SIRT1-Mediated NF-kappaB Pathway. Mar. Drugs 2022, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ruan, Y.; Xiong, C.; Xu, Q.; Wei, P.; Ma, P.; Bai, X.; Du, Y. Chitosan oligosaccharides suppressant LPS binding to TLR4/MD-2 receptor complex. Carbohydr. Polym. 2010, 82, 405–411. [Google Scholar] [CrossRef]











| Gene | Primers |
|---|---|
| Irak4 | F: AACAGACGGCCAGACATT R: CAACAGGTAGGTGACGCA |
| Map2k6 | F: ACTGTGGATTGGTGGGTT R: CCTCAGAAAGGCAGGGT |
| Tlr4 | F: CTTTGCTTCCTTGGTGTTG R: ATGATTCTCCTCTTCTTCACG |
| Cflar | F: CCGCAGGCTAACTTTCC R: CCCATCCCACTCAACAAC |
| Bcl-2 | F: AAACCCTCCATCCTGTCC R: TCCTAAACCCTGCTTCCC |
| Fadd | F: TGCTCCATCTGGCTGTT R: GCATAGTCTGGGGAGTCAA |
| Il-1r | F: GTGCGGGACACTAAGGAG R: TTTACTGGGAGGCACAGC |
| Cd14 | F: GACTCTGAATCCCACTCGGAGA R: TAGGAGCAAAGCCAGAGTTCCT |
| Cd40 | F: GACTGCTTGCTGACCTTTG F: GACTGCTTGCTGACCTTTG |
| Ddit3 | F: GAACAGTGGGCATCACCTC R: CAGTCCCCTCCTCAGCAT |
| H2-Dma | F: AATGCCCTGTGTGGTGTG R: GCCTGTGCTGTTCCAAGAT |
| Nos1 | F: AAAACACAGGAGCAAGCG R: TGGTTGGGTCAGCAATCT |
| Mpo | F: CCCGCATTCCTTGTTTTC R: CTTCTCCCCATTCCATCG |
| Tap1 | F: GTTGAGGAGCGGAGGTG R: TCAGTGTTGGCGTGAGGT |
| Sample | Raw Reads | Clean Reads | Clean Bases | Error (%) | Q20 (%) a | Q30 (%) a | GC (%) b |
|---|---|---|---|---|---|---|---|
| CK-1 | 1.27 × 108 | 1.27 × 108 | 19.06 G | 0.02 | 97.88 | 93.34 | 49.31 |
| CK-2 | 94,739,692 | 94,739,544 | 14.19 G | 0.02 | 97.96 | 93.43 | 49.05 |
| CK-3 | 1.11 × 108 | 1.11 × 108 | 16.64 G | 0.03 | 96.72 | 90.54 | 49.44 |
| LPS-1 | 79,670,242 | 79,670,150 | 11.93 G | 0.02 | 97.84 | 93.35 | 49.79 |
| LPS-2 | 91,538,486 | 91,538,360 | 13.7 G | 0.02 | 97.81 | 93.27 | 49.8 |
| LPS-3 | 97,241,286 | 97,241,092 | 14.56 G | 0.02 | 98.24 | 94.41 | 49.63 |
| EG-1 | 99,173,740 | 99,173,666 | 14.84 G | 0.03 | 97.14 | 91.57 | 50.25 |
| EG-2 | 1.28 × 108 | 1.28 × 108 | 19.22 G | 0.02 | 98.01 | 93.81 | 50.48 |
| EG-3 | 1.2 × 108 | 1.2 × 108 | 18.01 G | 0.03 | 97.7 | 92.97 | 50.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Li, K.; Liu, S.; Yu, H.; Li, P.; Xing, R. Pleiotropic Modulation of Chitooligosaccharides on Inflammatory Signaling in LPS-Induced Macrophages. Polymers 2023, 15, 1613. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15071613
Hao W, Li K, Liu S, Yu H, Li P, Xing R. Pleiotropic Modulation of Chitooligosaccharides on Inflammatory Signaling in LPS-Induced Macrophages. Polymers. 2023; 15(7):1613. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15071613
Chicago/Turabian StyleHao, Wentong, Kecheng Li, Song Liu, Huahua Yu, Pengcheng Li, and Ronge Xing. 2023. "Pleiotropic Modulation of Chitooligosaccharides on Inflammatory Signaling in LPS-Induced Macrophages" Polymers 15, no. 7: 1613. https://0-doi-org.brum.beds.ac.uk/10.3390/polym15071613