Luminescence and Magnetic Properties of Two Three-Dimensional Terbium and Dysprosium MOFs Based on Azobenzene-4,4′-Dicarboxylic Linker
Abstract
:1. Introduction

2. Experimental Section
2.1. General Procedures
2.2. Preparation of Complexes
2.3. Physical Measurements
2.4. Single-Crystal Structure Determination
| Compound | 1 |
|---|---|
| Chemical formula | C30H41N6O13Tb |
| M/gmol−1 | 852.61 |
| T(K) | 100(2) |
| λ/Å | 0.71073 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 9.7799(5) |
| b/Å | 11.5661(7) |
| c/Å | 16.7597(10) |
| α/deg | 105.886(2) |
| β/deg | 100.772(2) |
| γ/deg | 100.290(2) |
| V/Å3 | 1,737.46(17) |
| Z | 2 |
| ρcalcd (g·cm−3) | 1.630 |
| μ(mm−1) | 2.108 |
| R(int) | 0.0741 |
| GOF on F2 | 1.026 |
| R1 [I > 2σ(I)] | 0.0544 |
| wR2 [I > 2σ(I)] | 0.1173 |
2.5. Luminescence Measurements
2.6. Computational Calculations
2.7. Magnetic Measurements
3. Results and Discussion
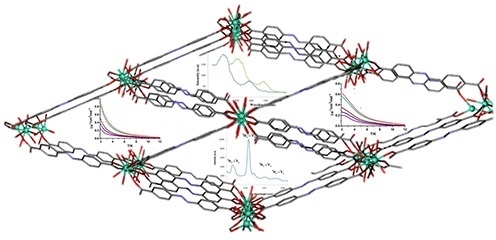
3.1. Description of the Structure


3.2. Adsorption Properties
3.3. Luminescence Studies


3.4. Magnetic Properties


4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farha, O.K.; Yazaydın, A.O.; Eryazici, I.; Malliakas, C.D.; Hauser, B.D.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Velazquez-Garcia, J.; Bennett, T.D.; Fairen-Jimenez, D. Mechanically and chemically robust ZIF-8 monoliths with high volumetric adsorption capacity. J. Mater. Chem. A 2015, 3, 2999–3005. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Almeida Paz, F.A.; Klinowski, J. Hydrothermal synthesis of a novel thermally stable three-dimensional ytterbium–organic framework. Chem. Commun. 2003, 13, 1484–1485. [Google Scholar] [CrossRef]
- Sun, D.; Cao, R.; Liang, Y.; Shi, Q.; Hong, M.J. Syntheses, crystal structures and properties of two novel lanthanide–carboxylate polymeric complexes. J. Chem. Soc. Dalton Trans. 2002, 8, 1847–1851. [Google Scholar] [CrossRef]
- Long, D.-L.; Blake, A.J.; Champness, N.R.; Wilson, C.; Schroeder, M. Unprecedented seven- and eight-connected lanthanide coordination networks. Angew. Chem. Int. Ed. 2001, 40, 2443–2447. [Google Scholar] [CrossRef]
- Westin, L.G.; Kritikos, M.; Caneschi, A. Self assembly, structure and properties of the decanuclear lanthanide ring complex, Dy10(OC2H4OCH3)30. Chem. Commun. 2003, 8, 1012–1013. [Google Scholar] [CrossRef]
- Wu, C.-D.; Lu, C.-Z.; Zhuang, H.-H.; Hang, J.-S. Hydrothermal assembly of a novel three-dimensional framework formed by [GdMo12O42]9− anions and nine coordinated GdIII cations. J. Am. Chem. Soc. 2002, 124, 3836–3837. [Google Scholar] [CrossRef]
- Ma, B.-Q.; Zhang, D.-S.; Gao, S.; Jin, T.-Z.; Yan, C.-H.; Xu, G.-X. From cubane to supercubane: The design, synthesis, and structure of a three-dimensional open framework based on a Ln4O4 cluster. Angew. Chem. Int. Ed. 2000, 39, 3644–3646. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wei, Y.-L.; Dong, X.-Y.; Zang, S.-Q.; Mak, T.C.W. Novel Tb-MOF embedded with viologen species for multi-photofunctionality: Photochromism, photomodulated fluorescence, and luminescent pH sensing. Chem. Mater. 2015, 27, 1327–1331. [Google Scholar] [CrossRef]
- Biswas, S.; Jena, H.S.; Goswami, S.; Sanda, S.; Konar, S. Synthesis and characterization of two lanthanide (Gd3+ and Dy3+)-based three-dimensional metal organic frameworks with squashed metallomacrocycle type building blocks and their magnetic, sorption, and fluorescence properties study. Cryst. Growth Des. 2014, 14, 1287–1295. [Google Scholar] [CrossRef]
- Baldov, J.J.; Coronado, E.; Gaita-Ariño, A.; Gamer, C.; Giménez-Marqués, M.; Mínguez-Espallargas, G. A SIM-MOF: Three-dimensional organisation of single-ion magnets with anion-exchange capabilities. Chem. Eur. J. 2014, 20, 10695–10702. [Google Scholar] [CrossRef] [PubMed]
- Briones, D.; Fernandez, B.; Calahorro, A.J.; Fairen-Jimenez, D.; Sanz, R.; Martínez, F.; Orcajo, G.; San Sebastian, E.; Seco, J.M.; Sánchez González, C.; et al. Highly active anti-diabetic metal-organic framework. Cryst. Growth Des. 2016. [Google Scholar] [CrossRef]
- Cuia, Y.; Chena, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273, 76–86. [Google Scholar] [CrossRef]
- Rodríguez-Diéguez, A.; Salinas-Castillo, A.; Sironi, A.; Seco, J.M.; Colacio, E. A chiral diamondoid 3D lanthanum metal–organic framework displaying blue-greenish long lifetime photoluminescence emission. Cryst. Eng. Comm. 2010, 12, 1876–1879. [Google Scholar] [CrossRef]
- Ruiz, J.; Mota, A.J.; Rodríguez-Diéguez, A.; Titos, S.; Herrera, J.M.; Ruiz, E.; Cremades, E.; Pierre Costes, J.; Colacio, E. Field and dilution effects on the slow relaxation of a luminescent DyO9 low-symmetry single-ion magnet. Chem. Commun. 2012, 48, 7916–7918. [Google Scholar] [CrossRef] [PubMed]
- Oyarzabal, I.; Ruiz, J.; Seco, J.M.; Evangelisti, M.; Camón, A.; Ruiz, E.; Aravena, D.; Colacio, E. Rational electrostatic design of easy-axis magnetic anisotropy in a ZnII–DyIII–ZnII single-molecule magnet with a high energy barrier. Chem. Eur. J. 2014, 20, 14262–14269. [Google Scholar] [CrossRef] [PubMed]
- Calahorro, A.J.; Oyarzabal, I.; Fernández, B.; Seco, J.M.; Tian, T.; Fairen-Jimenez, D.; Colacio, E.; Rodríguez-Diéguez, A. Rare earth anthracenedicarboxylate metal–organic frameworks: Slow relaxation of magnetization of Nd3+, Gd3+, Dy3+, Er3+ and Yb3+ based materials. Dalton Trans. 2016, 45, 591–598. [Google Scholar] [CrossRef]
- Zhuang, J.-L.; Lommel, K.; Ceglarek, D.; Andrusenko, I.; Kolb, U.; Maracke, S.; Sazama, U.; Froba, M.; Terfort, A. Synthesis of a new copper-azobenzene dicarboxylate framework in the form of hierarchical bulk solids and thin films without and with patterning. Chem. Mater. 2011, 23, 5366–5474. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Adsorption Correction; Institute for Inorganic Chemistry, University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELX97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON-94 (V-101094), a Multipurpose Crystallographic Tool; University of Utrecht: Utrecht, The Netherlands, 1994. [Google Scholar]
- Topas-R Software; General profile and structure analysis software for powder diffraction data; Bruker AXS: Brisbane, Australia, 2012.
- Gelb, L.D.; Gubbins, K.E. Pore size distributions in porous glasses: a computer simulation study. Langmuir 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Düren, T.; Millange, F.; Férey, G.; Walton, K.S.; Snurr, R.Q. Calculating geometric surface areas as a characterization tool for metal-organic frameworks. J. Phys. Chem. C 2007, 111, 15350–15356. [Google Scholar] [CrossRef]
- Seco, J.M.; Fairen-Jimenez, D.; Calahorro, A.J.; Mendez-Linan, L.; Perez-Mendoza, M.; Casati, N.; Colacio, E.; Rodriguez-Dieguez, A. Modular structure of a robust microporous MOF based on Cu2 paddle-wheels with high CO2 selectivity. Chem. Commun. 2013, 49, 11329–11331. [Google Scholar] [CrossRef] [PubMed]
- Feldblyum, J.I.; Liu, M.; Gidley, D.W.; Matzger, A.J. Reconciling the discrepancies between crystallographic porosity and guest access as exemplified by Zn-HKUST-1. J. Am. Chem. Soc. 2011, 133, 18257–18263. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.-D.; Zhao, B.; Liu, L.; Dai, J.-C. Synthesis, structure, and characterization of polymeric lanthanide 2-aminoterephthalate frameworks [Ln2(atp)3(H2O)2]·dmf·4H2O. Inorg. Chem. Comm. 2015, 61, 140–143. [Google Scholar] [CrossRef]
- Almáši, M.; Zeleňák, V.; Galdun, L.; Kuchár, J. First 3D coordination polymer built from Ho(III) and 2-aminoterephthalate ligand. Inorg. Chem. Commun. 2014, 39, 39–42. [Google Scholar] [CrossRef]
- Calahorro, A.J.; Peñas-Sanjuan, A.; Melguizo, M.; Fairén-Jiménez, D.; Zaragoza, G.; Fernández, B.; Salinas-Castillo, A.; Rodríguez-Diéguez, A. First examples of metal−organic frameworks with the novel 3,3′-(1,2,4,5-tetrazine-3,6-diyl)dibenzoic spacer. Luminescence and adsorption properties. Inorg. Chem. 2013, 52, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Shyni, R.; Biju, S.; Reddy, M.L.P.; Cowley, A.H.; Findlater, M. Synthesis, crystal structures, and photophysical properties of homodinuclear lanthanide xanthene-9-carboxylates. Inorg. Chem. 2007, 46, 11025–11030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Zhang, Y.L.; Dou, W.; Zhang, A.J.; Qin, W.W.; Liu, W.S. Synthesis, radii dependent self-assembly crystal structures and luminescent properties of rare earth (III) complexes with a tripodal salicylic derivative. Dalton Trans. 2010, 39, 9013–9021. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, B.; Oyarzabal, I.; Seco, J.M.; Sebastián, E.S.; Fairen-Jiménez, D.; Gómez-Ruiz, S.; Salinas-Castillo, A.; Calahorro, A.J.; Rodríguez-Diéguez, A. Luminescence and Magnetic Properties of Two Three-Dimensional Terbium and Dysprosium MOFs Based on Azobenzene-4,4′-Dicarboxylic Linker. Polymers 2016, 8, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8020039
Fernández B, Oyarzabal I, Seco JM, Sebastián ES, Fairen-Jiménez D, Gómez-Ruiz S, Salinas-Castillo A, Calahorro AJ, Rodríguez-Diéguez A. Luminescence and Magnetic Properties of Two Three-Dimensional Terbium and Dysprosium MOFs Based on Azobenzene-4,4′-Dicarboxylic Linker. Polymers. 2016; 8(2):39. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8020039
Chicago/Turabian StyleFernández, Belén, Itziar Oyarzabal, José M. Seco, Eider San Sebastián, David Fairen-Jiménez, Santiago Gómez-Ruiz, Alfonso Salinas-Castillo, Antonio J. Calahorro, and Antonio Rodríguez-Diéguez. 2016. "Luminescence and Magnetic Properties of Two Three-Dimensional Terbium and Dysprosium MOFs Based on Azobenzene-4,4′-Dicarboxylic Linker" Polymers 8, no. 2: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/polym8020039