Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
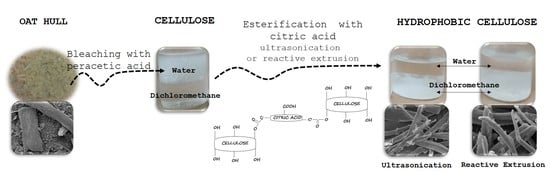
2.2. Extraction of Cellulose from Oat Hulls
2.3. Surface Modification of Cellulose
2.4. Characterization of Modified Cellulose
2.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.2. X-ray Diffraction (XRD)
2.4.3. Thermogravimetric Analysis (TGA)
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. Scanning Electron Microscopy (SEM)
2.4.6. Wettability
2.4.7. Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC)
2.4.8. Moisture Sorption Isotherms
2.5. Statistical Analysis
3. Results
3.1. Cellulose Extraction
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3. X-ray Diffraction (XRD)
3.4. Thermogravimetric Analysis (TGA)
3.5. Differential Scanning Calorimetry (DSC)
3.6. Scanning Electron Microscopy (SEM)
3.7. Wettability
3.8. Water Absorption Capacity (WAC) and Oil Absorption Capacity (OAC)
3.9. Moisture Sorption Isotherms
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef] [PubMed]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortéz-Gracía, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Liguoriç, R.; Facaro, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ganewatta, M.S.; Tang, C. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101, 101197. [Google Scholar] [CrossRef]
- De Corato, U.; De Bari, I.; Viola, E.; Pugliese, M. Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: A review, Renew. Renew. Sustain. Energy Rev. 2018, 88, 326–346. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processes based on reactive extrusion. Cellulose 2020, 27, 1975–1988. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A Green approach based on reactive extrusion to produce nanofibrillated cellulose from oat hull. Waste Biomass Valorization 2021, 12, 1051–1070. [Google Scholar] [CrossRef]
- Mantovan, J.; Giraldo, G.A.G.; Marim, B.M.; Kishima, J.O.G.; Mali, S. Valorization of orange bagasse through one-step physical and chemical combined processes to obtain a cellulose-rich material. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. Micro and nanocrystalline cellulose derivatives of lignocellulosic biomass: A review on synthesis, applications and advancements: A review on synthesis, applications and advancements. Carbohydr. Polym. 2020, 250, 116937. [Google Scholar] [CrossRef]
- Ge, H.; Huang, H.L.; Xu, M.; Chen, Q. Cellulose/poly (ethylene imine) composites as eficiente and reusable adsorbents for heavy metal ions. Cellulose 2016, 23, 2527–2537. [Google Scholar] [CrossRef]
- Mihaly-Cozmuta, A.; Peter, A.; Craciun, G.; Falup, A.; Mihaly-Cozmuta, L.; Nicula, C.; Vulpoi, A.; Baia, M. Preparation and characterization of active cellulose-based papers modified with TiO2, Ag and zeolite nanocomposites for bread packaging application. Cellulose 2017, 24, 3911–3928. [Google Scholar] [CrossRef]
- Souza, E.; Gottschalk, L.; Freitas-silva, O. Overview of Nanocellulose in Food Packaging. Recent Pat. Food Nutr. Agric. 2020, 11, 154–167. [Google Scholar] [CrossRef]
- Fischer, W.J.; Mayr, M.; Spirk, S.; Reishofer, D.; Jagiello, L.A.; Schmiedt, R.; Colson, J.; Zankel, A.; Bauer, W. Pulp fines characterization, sheet formation, and comparison to microfibrillated cellulose. Polymer 2017, 9, 366. [Google Scholar] [CrossRef]
- Marchetti, L.; Andrés, S.C. Use of nanocellulose in meat products. Curr. Opin. Food Sci. 2021, 38, 6–101. [Google Scholar] [CrossRef]
- Ma, T.; Hu, X.; Lu, S.; Liao, X.; Song, Y.; Hu, X. Nanocellulose: A promising green treasure from food wastes to available food materials. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.P.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Faria-Tischer, P.C.S.; Ribeiro-Viana, R.M.; Tischer, C.A. Bio-based nanocomposites: Strategies for cellulose functionalization and tissue affinity studies Materials for Biomedical Engineering. Biopolym. Fibers 2019, 205–244. [Google Scholar] [CrossRef]
- O’ConnelL, D.W.; Birkinshaw, C.; O’dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. cellulose regeneration and chemical recycling: Closing the “cellulose gap” using environmentally benign solvents. Macromol. Mater. Eng. 2020, 1900832. [Google Scholar] [CrossRef]
- Wang, Y.; Xiaojie, W.; Xie, Y.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S.A. Review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpaa, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: Innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- De Cuadro, P.; Belt, T.; Kontturi, K.S.; Reza, M.; Kontturi, E.; Vuorinen, T.; Hughes, M. Cross-linking of cellulose and poly (ethylene glycol) with citric acid. React. Funct. Polym. 2015, 90, 21–24. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Ghosh, P.; Gangopadhyay, R. Photofunctionalization of cellulose and lignocellulose fibres using photoactive organic acids. Eur. Polym. J. 2000, 36, 625–634. [Google Scholar] [CrossRef]
- He, X.; Luzi, F.; Yang, W.; Xiao, Z.; Torre, L.; Xie, Y.; Puglia, D. Citric acid as green modifier for tuned hydrophobicity of surface modified cellulose and lignin nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 9966–9978. [Google Scholar] [CrossRef]
- Li, B.; Dong, Y.; Li, L. Preparation and catalytic performance of Fe(III)-citric acid-modified cotton fiber complex as a novel cellulose fiber-supported heterogeneous photo-Fenton catalyst. Cellulose 2015, 2, 1295–1309. [Google Scholar] [CrossRef]
- Romeo, I.; Olivito, F.; Tursi, A.; Algieri, V.; Beneduci, A.; Chidichimo, G.; Maiuolo, L.; Sicilia, E.; De Nino, A. Totally green cellulose conversion into bio-oil and cellulose citrate using molten citric acid in an open system: Synthesis, characterization and computational investigation of reaction mechanisms. J. R. Soc. Chem. 2020, 10, 34738–34751. [Google Scholar] [CrossRef]
- Ramirez, J.A.A.; Fortunati, E.; Kenny, J.M.; Torre, L.; Foresti, M.L. Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 1358–1364. [Google Scholar] [CrossRef]
- Emam, H.E.; Shaheen, T.I. Investigation into the role of surface modification of cellulose nanocrystals with succinic anhydride in dye removal. J. Polym. Environ. 2019, 27, 2419–2427. [Google Scholar] [CrossRef]
- Sehaqui, H.; Zimmermann, T.; Tingaut, P. Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 2014, 21, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, H.; Li, X.; Gibril, M.E.; Yu, M. Chemical modification of cellulose by in situ reactive extrusion in ionic liquid. Carbohydr. Polym. 2014, 99, 126–131. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Ultra-sonication assisted cross-linking of cellulose polymers. Ultrason. Sonochem. 2018, 42, 567–576. [Google Scholar] [CrossRef]
- Bhandari, P.N.; Jones, D.D.; Hanna, M.A. Carboxymethylation of cellulose using reactive extrusion. Carbohydr. Polym. 2012, 87, 2246–2254. [Google Scholar] [CrossRef]
- Tang, L.; Huang, B.; Lu, Q.; Wang, S.; Ou, W.; Lin, W.; Chen, X. Ultrasonication-assisted manufacture of cellulose nanocrystals esterified with acetic acid. Bioresour. Technol. 2013, 127, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Calbourn, K.; Matuana, L.M. Functionalization of wood particles through a reactive extrusion process. J. Appl. Polym. Sci. 2006, 101, 3131–3142. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Gaugler, M.; Smith, D.A. Green route to modification of wood waste, cellulose and hemicellulose using reactive extrusion. Carbohydr. Polym. 2016, 136, 1238–1250. [Google Scholar] [CrossRef]
- Van Soest, P.J. Symposium on factors influencing the voluntary intake of herbage by ruminants: Voluntary intake in relation to chemical composition and digestibility. J. Anim. Sci. 1965, 24, 834–843. [Google Scholar] [CrossRef]
- TAPPI TEST METHOD T222 om-88, Acid-Insoluble Lignin in Wood and Pulp, in Tappi Test Methods; Tappi Press: Atlanta, GA, USA, 1999.
- Dong, X.; Dong, Y.; Jiang, M.; Wang, L.; Tong, J.; Zhou, J. Modification of microcrystalline cellulose by using soybean oil for Surface hydrophobization. Ind. Crop. Prod. 2013, 46, 301–303. [Google Scholar] [CrossRef]
- Dastidar, T.G.; Netravali, A.N. ‘Green’ crosslinking of native starches with malonic acid and their properties. Carbohydr. Polym. 2012, 90, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of nature cellulose using the x-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Rockland, L.B. Saturated salt solutions for static control of relative humidity between 5° and 40 °C. Anal. Chem. 1960, 32, 1375–1376. [Google Scholar] [CrossRef]
- Bizot, H. Using the GAB model to construct sorption isotherms. In Physical Properties of Foods; Jowitt, R., Escher, F., Hallistrom, B., Meffert, H.F.T., Spiess, W.E.L., Vos, G., Eds.; Applied Science Publishers: London, UK, 1984; pp. 27–41. [Google Scholar]
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr. Polym. 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Kaya, M. Super absorbent, light, and highly flame retardant cellulose-based aerogel crosslinked with citric acid. J. Appl. Polym. Sci. 2017, 45315, 1–9. [Google Scholar] [CrossRef]
- Adewuyi, A.; Pereira, F.V. Surface modification of cellulose isolated from Sesamun indicum underutilized seed: A means of enhancing cellulose hydrophobicity. J. Sci. Adv. Mater. Dev. 2017, 2, 326–332. [Google Scholar] [CrossRef]
- Miranda, M.I.G.; Bica, C.I.D.; Nachtigall, S.M.B.; Rehman, N.; Rosa, S.M.L. Kinetical thermal degradation study of maize straw and soybean hull celluloses by simultaneous DSC–TGA and MDSC techniques. Thermochim. Acta 2013, 565, 65–71. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.K.; Basak, R.K.; Rana, A.K. Study of the thermal behavior of alkali-treated jute fibers. J. Appl. Polym. Sci. 2002, 12, 2594–2599. [Google Scholar] [CrossRef]
- Orozco, R.S.; Hernández, P.B.; Morales, G.R.; Núñez, F.U.; Villafuerte, J.O.; Lugo, V.L.; Ramírez, N.F.; Díaz, C.E.B.; Vázquez, P.C. Characterization of lignocellulosic fruit waste as an alternative feedstock for bioethanol production. BioResources 2014, 9, 1873–1885. [Google Scholar]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Agu, O.S.; Tabil, L.G.; Dumonceaux, T. Microwave-assisted alkali pre-treatment, densification and enzymatic saccharification of canola straw and oat hull. Bioengineering 2017, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Paschoal, G.B.; Muller, C.M.O.; Carvalho, G.M.; Tischer, C.A.; Mali, S. Isolation and characterization of nanofibrillated cellulose from oat hulls. Quim. Nova 2015, 38, 478–482. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact angle and wettability of cellulosic surfaces: A review of proposed mechanisms and test strategies. Bioresources 2015, 10, 8657–8749. [Google Scholar] [CrossRef] [Green Version]
- Da Conceição, I.D.; Da Silva, L.R.C.; Alves, T.S.; E Silva, H.S.; Barbosa, R.; DE Sousa, R.R.M. investigation of the wettability using contact angle measurements of green polyethylene flat films and expanded vermiculite clay treated by plasma. Mater. Res. 2019, 22, 20180918. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Qian, Q.; Hou, Z.; Lui, Y.; Lu, M. Preparation of smart and reversible wettability cellulose fabrics for oil/water separation using a facile and economical method. Carbohydr. Polym. 2018, 200, 63–71. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Plasticizers in edible films and coatings. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: Cambridge, UK, 2005; pp. 403–433. [Google Scholar] [CrossRef]








| Sample | TGA Parameters | ||
|---|---|---|---|
| Tmax1 (°C) | Tmax2 (°C) | T10 (°C) | |
| Cellulose | 360 | 435 | 457 |
| US401minCA | 348 | 468 | 478 |
| US402minCA | 349 | 469 | 470 |
| US801minCA | 350 | 468 | 478 |
| US802minCA | 350 | 463 | 479 |
| ECW | 355 | 449 | 460 |
| EMCCA | 360 | 462 | 473 |
| Sample | WAC (g/g) | OAC (g/g) |
|---|---|---|
| Cellulose | 9.38 ± 0.01 a | 1.80 ± 0.01 d |
| US401minCA | 1.69 ± 0.01 c | 6.78 ± 0.30 a,b |
| US402minCA | 1.68 ± 0.01 c | 6.73 ± 0.30 a,b |
| US801minCA | 1.70 ± 0.01 c | 6.59 ± 0.04 b |
| US802minCA | 1.71 ± 0.01 c | 7.31 ± 0.12 a |
| ECW | 7.75 ± 0.01 b | 2.04 ± 0.50 d |
| EMCCA | 1.63 ± 0.01 c | 4.57 ± 0.32 c |
| Sample | m0 | C | K | r2 |
|---|---|---|---|---|
| Cellulose | 39.19 | 1.44 | 0.23 | 0.99 |
| US401minCA | 11.78 | 1.71 | 0.40 | 0.98 |
| US402minCA | 20.42 | 1.53 | 0.26 | 0.98 |
| US801minCA | 16.86 | 1.54 | 0.31 | 0.98 |
| US802minCA | 19.11 | 1.47 | 0.28 | 0.98 |
| ECW | 16.67 | 1.92 | 0.39 | 0.99 |
| EMCCA | 5.68 | 2.68 | 0.55 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Mali, S. Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes. Polysaccharides 2021, 2, 218-233. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2020015
Gil Giraldo GA, Mantovan J, Marim BM, Kishima JOF, Mali S. Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes. Polysaccharides. 2021; 2(2):218-233. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2020015
Chicago/Turabian StyleGil Giraldo, Gina Alejandra, Janaina Mantovan, Beatriz M. Marim, João Otávio F. Kishima, and Suzana Mali. 2021. "Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes" Polysaccharides 2, no. 2: 218-233. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2020015