Polysaccharide-Based Packaging Functionalized with Inorganic Nanoparticles for Food Preservation
Abstract
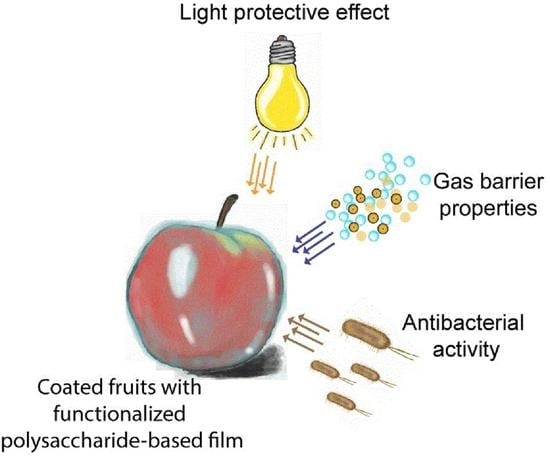
:1. Introduction
2. Polysaccharides as Food Packaging Materials
3. Functionalization of Polysaccharide-Based Materials for Food Packaging
4. Food Preservation Using Polysaccharide Packaging Functionalized with Inorganic Nanoparticles
4.1. Zinc Oxide (ZnO)
4.2. Titanium Dioxide (TiO2)
4.3. Silver (Ag)
4.4. Silicon Dioxide (SiO2)
4.5. Other Inorganic Nanomaterials Used to Develop Polysaccharide-Hybrid Packaging for Food Preservation
4.5.1. Halloysite (Hal)
4.5.2. Aluminum Oxide (Al2O3)
4.5.3. Montmorillonite (MMT)
4.5.4. Iron(III) Oxide (Fe2O3)
4.5.5. Zirconium (Zr4+)
4.5.6. Magnesium Oxide (MgO)
5. Disadvantages of Polysaccharide-Based Food Packaging Functionalized with Inorganic Nanoparticles and Perspectives
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vilela, C.; Pinto, R.; Pinto, S.; Marques, P.; Silvestre, A.; Freire, C. Polysaccharide Based Hybrid. Materials Metals and Metal Oxides, Graphene and Carbon Nanotubes; Springer: Berlin, Germany, 2014; Volume 53, ISBN 9783030003463. [Google Scholar]
- Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Ruvalcaba-Gómez, J.M.; Romero-Toledo, R.; Sandoval-Contreras, T.; Aguilera-Aguirre, S.; Montalvo-González, E.; Pérez-Larios, A. Use of titanium dioxide (TiO2) nanoparticles as reinforcement agent of polysaccharide-based materials. Processes 2020, 8, 1395. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A versatile hybrid composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, A.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Mousavi, A.; Razavi, B.; Shahi, S. Preparation of organic-inorganic hybrid nanocomposites from chemically modified epoxy and novolac resins and silica-attached carbon nanotubes by sol-gel process: Investigation of thermal degradation and stability. Prog. Org. Coat. 2018, 117, 154–165. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for food packaging applications: An overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Montalvo-González, E.; González-Silva, N.; Méndez-Robles, M.D.; Romero-Toledo, R.; Yahia, E.M.; Pérez-Larios, A. Synthesis and characterization of TiO2-ZnO-MgO mixed oxide and their antibacterial activity. Materials 2019, 12, 698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Nahida, J.H. SiO2 particles effect on the mechanical properties of the starch/PVA blends. Iraqi J. Phys. 2018, 16, 153–171. [Google Scholar]
- Alebooyeh, R.; MohammadiNafchi, A.; Jokar, M. The effects of ZnO nanorods on the characteristics of sago starch biodegradable films. J. Chem. Health Risks 2012, 2, 13–16. [Google Scholar]
- Saedi, S.; Rhim, J.W. Synthesis of Fe3O4@SiO2@PAMAM dendrimer@AgNP hybrid nanoparticles for the preparation of carrageenan-based functional nanocomposite film. Food Packag. Shelf Life 2020, 24, 100473. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Pérez-Cabrera, L.E.; Artés-Hernández, F.; Martínez-Hernández, G.B. Ag-chitosan nanocomposites in edible coatings affect the quality of fresh-cut melon. Postharvest Biol. Technol. 2019, 147, 174–184. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; El-Sayed, H.S.; Ibrahim, O.A.; Youssef, A.M. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on roselle calyx extract for Ras cheese coating. Carbohydr. Polym. 2020, 239, 116234. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Scheibel, J.M.; Werner, J.O.; Brandelli, A. Starch-halloysite nanocomposites containing nisin: Characterization and inhibition of Listeria monocytogenes in soft cheese. LWT-Food Sci. Technol. 2016, 68, 226–234. [Google Scholar] [CrossRef]
- Osman, A.G.; El-Desouky, A.I.; Morsy, M.K.; Aboud, A.A.; Mohamed, M.H. Impact of aluminum oxide and silica oxide nanocomposite on foodborne pathogens in chicken fillets. Eur. J. Nutr. Food Saf. 2019, 9, 152–162. [Google Scholar] [CrossRef]
- Fayaz, M.A.; Balaji, K.; Girilal, M.; Kalaichelvan, P.T.; Venkatesan, R. Mycobased synthesis of silver nanoparticles and their incorporation into sodium alginate films for vegetable and fruit preservation. J. Agric. Food Chem. 2009, 57, 6246–6252. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Regenstein, J.M.; Kafil, H.S. Protection of foods against oxidative deterioration using edible films and coatings: A review. Food Biosci. 2019, 32, 100451. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2020, 25, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M.; Durairaj, R.B.; Nageshwaran, G.; Joseph, G.B. A brief review on edible food packaging materials. J. Glob. Eng. Probl. Solut. 2017, 1, 9–19. [Google Scholar]
- Jeevahan, J.J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Joseph, G.B.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Stefanowski, J.; Weiss, D. Carrot2 and language properties in web search results clustering. Lect. Notes Artif. Intell. 2003, 2663, 240–249. [Google Scholar]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ishikawa, K.; Shirosaki, Y.; Ohtsuki, C. Bioceramic research on intelligent implants and drug delivery system organic–inorganic composites designed for biomedical applications. Biol. Pharm. Bull. 2013, 36, 1670–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedi, Y.; Fathi-Achachlouei, B.; Yousefi, A.R. Physical and mechanical properties of hybrid montmorillonite/zinc oxide reinforced carboxymethyl cellulose nanocomposites. Int. J. Biol. Macromol. 2018, 108, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.Y.; Patanen, M.; Sirviö, J.A.; Visanko, M.; Ohigashi, T.; Kosugi, N.; Huttula, M.; Liimatainen, H. Hybrid films of cellulose nanofibrils, chitosan and nanosilica: Structural, thermal, optical, and mechanical properties. Carbohydr. Polym. 2019, 218, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortiz, M.A.; Palma-Rodríguez, H.M.; Montalvo-González, E.; Sáyago-Ayerdi, S.G.; Utrilla-Coello, R.; Vargas-Torres, A. Effect of using microencapsulated ascorbic acid in coatings based on resistant starch chayotextle on the quality of guava fruit. Sci. Hortic. 2019, 256, 108604. [Google Scholar] [CrossRef]
- Kanikireddy, V.; Varaprasad, K.; Rani, M.S.; Venkataswamy, P.; Mohan Reddy, B.J.; Vithal, M. Biosynthesis of CMC-Guar gum-Ag0 nanocomposites for inactivation of food pathogenic microbes and its effect on the shelf life of strawberries. Carbohydr. Polym. 2020, 236, 116053. [Google Scholar] [CrossRef]
- Dias, M.V.; De Fátima, F.; Soares, N.; Borges, S.V.; De Sousa, M.M.; Nunes, C.A.; De Oliveira, I.R.N.; Medeiros, E.A.A. Use of allyl isothiocyanate and carbon nanotubes in an antimicrobial film to package shredded, cooked chicken meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Deng, J.; Jiao, J.; Lu, Y.; Yang, L.; Shi, Z. The combined effects of carboxymethyl chitosan and Cryptococcus laurentii treatment on postharvest blue mold caused by Penicillium italicum in grapefruit fruit. Sci. Hortic. 2019, 253, 35–41. [Google Scholar] [CrossRef]
- Ruelas-Chacon, X.; Aguilar-González, A.; Reyes-Vega, M. de la L.; Peralta-Rodríguez, R.D.; Corona-Flores, J.; Rebolloso-Padilla, O.N.; Aguilera-Carbo, A.F. Bioactive protecting coating of guar gum with thyme oil to extend shelf life of tilapia (Oreoschromis niloticus) fillets. Polymers 2020, 12, 3019. [Google Scholar] [CrossRef] [PubMed]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Bozkurt, F.; Goudjil, M.B.; Tornuk, F. Home-made cheese preservation using sodium alginate based on edible film incorporating essential oils. J. Food Sci. Technol. 2020, 58, 2406–2419. [Google Scholar] [CrossRef] [PubMed]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; Rosa, G.S.; Dotto, G.L. Bio-based active packaging: Carrageenan film with olive leaf extract for lamb meat preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Keshk, S.M.A.S.; El-Zahhar, A.A.; Haija, M.A.; Bondock, S. Synthesis of a magnetic nanoparticles/dialdehyde starch-based composite film for food packaging. Starch/Staerke 2019, 71, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Tunma, S. Starch based nanocomposites in active packaging for extended shelf life of fresh fruits. Walailak J. Sci. Technol. 2018, 15, 273–281. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Anugrah, D.S.B.; Alexander, H.; Pramitasari, R.; Hudiyanti, D.; Sagita, C.P. A review of polysaccharide-zinc oxide nanocomposites as safe coating for fruits preservation. Coatings 2020, 10, 988. [Google Scholar] [CrossRef]
- Ebrahimiasl, S.; Zakaria, A.; Kassim, A.; Basri, S.N. Novel conductive polypyrrole/zinc oxide/chitosan bionanocomposite: Synthesis, characterization, antioxidant, and antibacterial activities. Int. J. Nanomed. 2015, 10, 217–227. [Google Scholar]
- Nain, V.; Kaur, M.; Sandhu, K.S.; Thory, R.; Sinhmar, A. Development, characterization, and biocompatibility of zinc oxide coupled starch nanocomposites from different botanical sources. Int. J. Biol. Macromol. 2020, 162, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, P.; Beltrame, E.; Zanet, V.; Vidic, J.; Manzano, M. Development and evaluation of qPCR detection method and Zn-MgO/alginate active packaging for controlling Listeria monocytogenes contamination in cold-smoked salmon. Foods 2020, 9, 1353. [Google Scholar] [CrossRef]
- Lavinia, M.; Hibaturrahman, S.N.; Harinata, H.; Wardana, A.A. Antimicrobial activity and application of nanocomposite coating from chitosan and ZnO nanoparticle to inhibit microbial growth on fresh-cut papaya. Food Res. 2020, 4, 307–311. [Google Scholar] [CrossRef]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dutta, J.; Dobretsov, S. Nanocomposite zinc oxide-chitosan coatings on polyethylene films for extending storage life of okra (Abelmoschus esculentus). Nanomaterials 2018, 8, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional betanin nanoliposomes-incorporated gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film for fresh beef preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef]
- Amjadi, S.; Emaminia, S.; Nazari, M.; Davudian, S.H.; Roufegarinejad, L.; Hamishehkar, H. Application of reinforced ZnO nanoparticle-incorporated gelatin bionanocomposite film with chitosan nanofiber for packaging of chicken fillet and cheese as food models. Food Bioprocess Technol. 2019, 12, 1205–1219. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Guo, X.; Chen, B.; Wu, X.; Li, J.; Sun, Q. Utilization of cinnamaldehyde and zinc oxide nanoparticles in a carboxymethylcellulose-based composite coating to improve the postharvest quality of cherry tomatoes. Int. J. Biol. Macromol. 2020, 160, 175–182. [Google Scholar] [CrossRef]
- Koushesh Saba, M.; Amini, R. Nano-ZnO/carboxymethyl cellulose-based active coating impact on ready-to-use pomegranate during cold storage. Food Chem. 2017, 232, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, K.B. Antimicrobial activity of buckwheat starch films containing zinc oxide nanoparticles against Listeria monocytogenes on mushrooms. Int. J. Food Sci. Technol. 2018, 53, 1549–1557. [Google Scholar] [CrossRef]
- Emamifar, A.; Bavaisi, S. Nanocomposite coating based on sodium alginate and nano-ZnO for extending the storage life of fresh strawberries (Fragaria × ananassa Duch.). J. Food Meas. Charact. 2020, 14, 1012–1024. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Meindrawan, B.; Suyatma, N.E.; Wardana, A.A.; Pamela, V.Y. Nanocomposite coating based on carrageenan and ZnO nanoparticles to maintain the storage quality of mango. Food Packag. Shelf Life 2018, 18, 140–146. [Google Scholar] [CrossRef]
- Romadhan, M.F.; Pujilestari, S. Synthesis of ZnO nanoparticles and their application as edible coatings based on pectin to extend the shelf life of Averrhoa carambola. J. Agroindustri Halal 2019, 5, 30–38. [Google Scholar]
- Baek, S.K.; Song, K.B. Development of Gracilaria vermiculophylla extract films containing zinc oxide nanoparticles and their application in smoked salmon packaging. LWT Food Sci. Technol. 2018, 89, 269–275. [Google Scholar] [CrossRef]
- Datta, J.; Kosiorek, P.; Włoch, M. Effect of high loading of titanium dioxide particles on the morphology, mechanical and thermo-mechanical properties of the natural rubber-based composites. Iran. Polym. J. 2016, 25, 1021–1035. [Google Scholar] [CrossRef] [Green Version]
- Anaya-Esparza, L.M.; González-Silva, N.; Yahia, E.M.; González-Vargas, O.A.; Montalvo-González, E.; Pérez-Larios, A. Effect of TiO2-ZnO-MgO mixed oxide on microbial growth and toxicity against Artemia salina. Nanomaterials 2019, 9, 992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleyaei, S.A.; Zahedi, Y.; Ghanbarzadeh, B.; Moayedi, A.A. Modification of physicochemical and thermal properties of starch films by incorporation of TiO2 nanoparticles. Int. J. Biol. Macromol. 2016, 89, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Goetz, N.V. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Qiao, G.; Xiao, Z.; Ding, W.; Rok, A. Effect of chitosan/nano-titanium dioxide/thymol and tween films on ready-to-eat cantaloupe fruit quality. Coatings 2019, 9, 828. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Wang, Q.; Li, W.; Li, X.; Shui, Y.; et al. Effect of chitosan/nano-TiO2 composite coatings on the postharvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109135. [Google Scholar] [CrossRef]
- Xu, W.; Xie, W.; Huang, X.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2016, 221, 267–277. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, M.; Li, X. Effects of Chitosan/TiO2 composite coating on keeping-fresh of stauntonvine. Adv. Mater. Res. 2012, 530, 68–73. [Google Scholar] [CrossRef]
- Sami, R.; Elhakem, A.; Alharbi, M.; Benajiba, N.; Almatrafi, M.; Jing, J.; Helal, M. Effect of titanium dioxide nanocomposite material and antimicrobial agents on mushrooms shelf-life preservation. Processes 2020, 8, 1632. [Google Scholar] [CrossRef]
- Tian, F.; Chen, W.; Wu, C.E.; Kou, X.; Fan, G.; Li, T.; Wu, Z. Preservation of Ginkgo biloba seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films. Int. J. Biol. Macromol. 2019, 126, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Process. Preserv. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-sani, M.; Mohammadian, E.; Julian, D. Eco-friendly active packaging consisting of nanostructured biopolymer matrix reinforced with TiO2 and essential oil: Application for preservation of refrigerated meat. Food Chem. 2020, 322, 126782. [Google Scholar] [CrossRef]
- Abdel Baky, E.; El -Duma Abdullah, Z.; El Din Aboul-Anean, H. Application of nano edible films to improve some Dates in Saudi Arabia. Int. J. Pharm. Res. Allied Sci. 2020, 9, 69–84. [Google Scholar]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Kyzioł, A.; Stochel, G. Development of noncytotoxic silver-chitosan nanocomposites for efficient control of biofilm forming microbes. RSC Adv. 2017, 7, 52398–52413. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.W.; Wang, L.F.; Hong, S.I. Preparation and characterization of agar/silver nanoparticles composite films with antimicrobial activity. Food Hydrocoll. 2013, 33, 327–335. [Google Scholar] [CrossRef]
- Ferraris, M.; Ferraris, S.; Miola, M.; Perero, S.; Balagna, C.; Verné, E.; Gautier, G.; Manfredotti, C.; Battiato, A.; Vittone, E.; et al. Effect of thermal treatments on sputtered silver nanocluster/ silica composite coatings on soda-lime glasses: Ionic exchange and antibacterial activity. J. Nanoparticle Res. 2012, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hasan, I.; Khan, R.A.; Alharbi, W.; Alharbi, K.H.; Abu Khanjer, M.; Alslame, A. Synthesis, characterization and photo-catalytic activity of guar-gum-g-aliginate@silver bionanocomposite material. RSC Adv. 2020, 10, 7898–7911. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Heli, B.; Morales-Narváez, E.; Golmohammadi, H.; Ajji, A.; Merkoçi, A. Modulation of population density and size of silver nanoparticles embedded in bacterial cellulose: Via ammonia exposure: Visual detection of volatile compounds in a piece of plasmonic nanopaper. Nanoscale 2016, 8, 7984–7991. [Google Scholar] [CrossRef] [PubMed]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [Green Version]
- Reddy, J.P.; Varada Rajulu, A.; Rhim, J.W.; Seo, J. Mechanical, thermal, and water vapor barrier properties of regenerated cellulose/nano-SiO2 composite films. Cellulose 2018, 25, 7153–7165. [Google Scholar] [CrossRef]
- Piroonpan, T.; Huajaikaew, E.; Katemake, P.; Pasanphan, W. Surface modification of SiO2 nanoparticles with PDMAEMA brushes and Ag nanoparticles as antifungal coatings using electron beam assisted synthesis. Mater. Chem. Phys. 2020, 253, 123438. [Google Scholar] [CrossRef]
- Ge, X.; Chu, M.; Qu, L.; Zhang, J.; Li, M.; Li, W.; Yao, Z. Long-lasting intrinsic polyethylene antifogging films generated by incorporating SiO2 nanoparticles into covalently grafted antifog agents. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 57, 826–836. [Google Scholar] [CrossRef]
- Hou, X.; Xue, Z.; Xia, Y.; Qin, Y.; Zhang, G.; Liu, H.; Li, K. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int. J. Biol. Macromol. 2019, 125, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Xia, Y. Preparation and characterization of nano-SiO2 reinforced alginate-based nanocomposite films (II). J. Appl. Polym. Sci. 2017, 134, 45286. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and characterization of potato starch film with various size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Li, B.; Chu, J.; Zhang, P. Silica in situ enhanced PVA/chitosan biodegradable films for food packages. Carbohydr. Polym. 2018, 184, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Eldib, R.; Khojah, E.; Elhakem, A.; Benajiba, N.; Helal, M. Chitosan, nisin, silicon dioxide nanoparticles coating films effects on blueberry (Vaccinium myrtillus) quality. Coatings 2020, 10, 962. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Alagu, T.; Karuppasamy, P.; Anbuganthi, P.; Lingasamy, P.; Jeyram, R.; Kuppamuthu, K.; Soundararajan, N. Synthesis and impregnation of Fe2O3 nanoparticles on cellulose paper and sodium alginate films for the preservation of fruit and vegetables. J. Microbiol. Biotechnol. Food Sci. 2020, 9, 1166–1169. [Google Scholar] [CrossRef]
- Ejeromedoghene, O.; Alayande, O.J.; Olatunji, E.D.; Alli, Y.A.; Adewuyi, S. Synthesis of Chitosan-Zirconium(IV) complexes as an antifungal spraying agent against tomato infected Aspergillus Niger. J. Chem. Soc. Niger. 2019, 44, 125–129. [Google Scholar]
- Naeeji, N.; Shahbazi, Y.; Shavisi, N. Effect of gamma irradiation on physico-mechanical and structural properties of basil seed mucilage-chitosan films containing Ziziphora clinopodioides essential oil and MgO nanoparticles for rainbow trout packaging. J. Food Process. Preserv. 2020, 44, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Tu, Y.; Zhao, Y. Gelatin-based nanocomposite film with bacterial cellulose-MgO nanoparticles and its application in packaging of preserved eggs. Coatings 2021, 11, 39. [Google Scholar] [CrossRef]
- Wang, L.F.; Rhim, J.W. Functionalization of halloysite nanotubes for the preparation of carboxymethyl cellulose-based nanocomposite films. Appl. Clay Sci. 2017, 150, 138–146. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Werner, J.O.; Brandelli, A. A novel active packaging material based on starch-halloysite nanocomposites incorporating antimicrobial peptides. Food Hydrocoll. 2017, 63, 561–570. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Biodegradable and water resistant poly(vinyl) alcohol (PVA)/starch (ST)/glycerol (GL)/halloysite nanotube (HNT) nanocomposite films for sustainable food packaging. Front. Mater. 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Devi, N.; Dutta, J. Development and in vitro characterization of chitosan/starch/halloysite nanotubes ternary nanocomposite films. Int. J. Biol. Macromol. 2019, 127, 222–231. [Google Scholar] [CrossRef]
- He, Y.; Kong, W.; Wang, W.; Liu, T.; Liu, Y.; Gong, Q.; Gao, J. Modified natural halloysite/potato starch composite films. Carbohydr. Polym. 2012, 87, 2706–2711. [Google Scholar] [CrossRef]
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Lazzara, G. Polysaccharides/Halloysite nanotubes for smart bionanocomposite materials. Carbohydr. Polym. 2020, 245, 116502. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Ju, J.; Yan, H.; Huang, X.; Tan, Y. Advances in halloysite nanotubes-polysaccharide nanocomposite preparation and applications. Polymers 2019, 11, 987. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Kim, S.Y.; Park, H.J. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocoll. 2018, 84, 58–67. [Google Scholar] [CrossRef]
- Hayes, K.L.; Mui, J.; Song, B.; Sani, E.S.; Eisenman, S.W.; Sheffield, J.B.; Kim, B. Effects, uptake, and translocation of aluminum oxide nanoparticles in lettuce: A comparison study to phytotoxic aluminum ions. Sci. Total Environ. 2020, 719, 137393. [Google Scholar] [CrossRef] [PubMed]
- Valerini, D.; Tammaro, L.; Di Benedetto, F.; Vigliotta, G.; Capodieci, L.; Terzi, R.; Rizzo, A. Aluminum-doped zinc oxide coatings on polylactic acid films for antimicrobial food packaging. Thin Solid Films 2018, 645, 187–192. [Google Scholar] [CrossRef]
- Llanos, J.H.R.; Tadini, C.C. Preparation and characterization of bio-nanocomposite films based on cassava starch or chitosan, reinforced with montmorillonite or bamboo nanofibers. Int. J. Biol. Macromol. 2018, 107, 371–382. [Google Scholar] [CrossRef]
- Cui, R.; Yan, J.; Cao, J.; Qin, Y.; Yuan, M.; Li, L. Release properties of cinnamaldehyde loaded by montmorillonite in chitosan-based antibacterial food packaging. Int. J. Food Sci. Technol. 2020, 1–12. [Google Scholar]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef]
- Lee, H.; Rukmanikrishnan, B.; Lee, J. Rheological, morphological, mechanical, and water-barrier properties of agar/gellan gum/montmorillonite clay composite films. Int. J. Biol. Macromol. 2019, 141, 538–544. [Google Scholar] [CrossRef]
- Yu, W.X.; Wang, Z.W.; Hu, C.Y.; Wang, L. Properties of low methoxyl pectin-carboxymethyl cellulose based on montmorillonite nanocomposite films. Int. J. Food Sci. Technol. 2014, 49, 2592–2601. [Google Scholar] [CrossRef]
- Yadav, M. Study on thermal and mechanical properties of cellulose/iron oxide bionanocomposites film. Compos. Commun. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Rashidzadeh, B.; Shokri, E.; Mahdavinia, G.R.; Moradi, R.; Mohamadi-Aghdam, S.; Abdi, S. Preparation and characterization of antibacterial magnetic-/pH-sensitive alginate/Ag/Fe3O4 hydrogel beads for controlled drug release. Int. J. Biol. Macromol. 2020, 154, 134–141. [Google Scholar] [CrossRef]
- Long, J.; Xu, E.; Li, X.; Wu, Z.; Wang, F.; Xu, X.; Jin, Z.; Jiao, A.; Zhan, X. Effect of chitosan molecular weight on the formation of chitosan-pullulanase soluble complexes and their application in the immobilization of pullulanase onto Fe3O4-κ-carrageenan nanoparticles. Food Chem. 2016, 202, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.; Pagar, T.; Ghotekar, S.; Pagar, K.; Pansambal, S. A Review on plant extract mediated green synthesis of zirconia nanoparticles and their miscellaneous applications. J. Chem. Rev. 2019, 1, 154–163. [Google Scholar]
- Bumajdad, A.; Nazeer, A.A.; Al Sagheer, F.; Nahar, S.; Zaki, M.I. Controlled Synthesis of ZrO2 nanoparticles with tailored size, morphology and crystal phases via organic/inorganic hybrid films. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, A.; Bhatia, N. Microwave assisted sol-gel synthesis of magnesium oxide (MgO). Int. J. Eng. Res. Develop. 2017, 13, 1–6. [Google Scholar]
- Tamilselvi, P.; Yelilarasi, A.; Hema, M.; Anbarasan, R. Synthesis of hierarchical structured MgO by sol-gel method. Nano Bull. 2013, 2, 130106. [Google Scholar]
- Rezaei, M.; Khajenoori, M.; Nematollahi, B. Synthesis of high surface area nanocrystalline MgO by pluronic P123 triblock copolymer surfactant. Powder Technol. 2011, 205, 112–116. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Kishore, N. Preparation and characterization of MgO nanoparticles by co-precipitation method. Int. J. Eng. Appl. Man. Sci. Paradig. 2013, 7, 66–70. [Google Scholar]
- Enescu, D.; Dehelean, A.; Gonçalves, C.; Cerqueira, M.A.; Magdas, D.A.; Fucinos, P.; Pastrana, L.M. Evaluation of the specific migration according to EU standards of titanium from chitosan/metal complexes films containing TiO2 particles into different food simulants. A comparative study of the nano-sized vs micro-sized particles. Food Packag. Shelf Life 2020, 26, 100579. [Google Scholar] [CrossRef]
- Fortunati, E.; Peltzer, M.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Garcia, C.V.; Shin, G.H.; Kim, J.T. Metal oxide-based nanocomposites in food packaging: Applications, migration, and regulations. Trends Food Sci. Technol. 2018, 82, 21–31. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Kumar, P.; Gautam, S. Developing ZnO Nanoparticle embedded antimicrobial starch biofilm for food packaging. arXiv 2019, arXiv:1909.05083. [Google Scholar]
- Zhang, B.; Luo, Y.; Wang, Q. Development of silver-zein composites as a promising antimicrobial agent. Biomacromolecules 2010, 11, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Mohammadzadeh Pournasir, N.; Pakdel, P. Properties of active starch-based films incorporating a combination of Ag, ZnO and CuO nanoparticles for potential use in food packaging applications. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Abdullah, Z.W.; Dong, Y. Preparation and characterisation of poly(vinyl) alcohol (PVA)/starch (ST)/halloysite nanotube (HNT) nanocomposite films as renewable materials. J. Mater. Sci. 2018, 53, 3455–3469. [Google Scholar] [CrossRef] [Green Version]




| Polysaccharide | Functional Agent | Presentation | Food Product | Ref. |
|---|---|---|---|---|
| Starch | Ascorbic acid | Edible film | Guava fruit | [27] |
| Carboxymethyl cellulose | Guar gum | Edible film | Strawberry fruit | [28] |
| Cellulose | Allyl isothiocyanate | Edible film | Chicken breast meat | [29] |
| Chitosan | Cryptococcus laurentii | Edible film | Grapefruit fruit | [30] |
| Guar gum | Thyme oil | Edible film | Tilapia fillets | [31] |
| Sodium alginate | Rosmarinus officinalis essential oil | Edible film | Soft cheese | [32] |
| K-Carrageenan | Olive leaf extract | Edible film | Lamb meat | [33] |
| Pectin | Oregano essential oil | Edible film | Pork loin | [34] |
| Polysaccharide | ZnO Specifications | Other Additives | Coating Method/Presentation | Food Product | Storage Conditions | Observed Results | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan (3 g in 300 mL) | Conc.: 0.027% w/w Commercial Size: 600 nm | Acetic acid (1 mL/100 mL) | Dipping for 10–20 s and drained at 25 °C/film | Fresh-cut papaya | 10 °C for 12 days | Treated fruit showed a reduced microbial growth. | [43] |
| Chitosan (5% w/v) | Conc.: 1% Commercial | Sodium alginate (10% w/w in chitosan weight) Glycerol (2% v/v) | Dipping/film | Guava | 21 °C for 20 days at 80% RH | The composite delayed the ripening process without apparent lesions. | [44] |
| Chitosan (2%) | Commercial Size: 35–45 nm | NI | Evaporative casting/coating | Okra | 25 °C for 12 days | The treated product showed reduced the fungal and bacterial growth. | [45] |
| Chitosan (1.4 w/v, DD 80–95%) | Commercial Size: 30 nm | Linseed oil 1:2 ratio on a potato protein solution (1.2%) glycerol (1.5%) | Evaporative casting/coating | Raw meat | 4 °C for 7 days | Threated meat preserved its sensory properties. | [46] |
| Chitosan (0.4 g in 100 mL) | Conc.: 0.2% w/v | The betanin-loaded NLPs (10%) gelatin (4 g/100 mL) glycerol (1 g/100 mL) | Evaporative casting/coating | Fresh beef | 4 °C for 16 days | Treated meat exhibited a reduced physicochemical changes during storage. | [47] |
| Chitosan (10% w/v) | Commercial Size: 30 nm | Gelatin (4% w/v), glycerol (25%) | Evaporative casting/coating | Chicken fillet | 4 °C for 12 days at 80% RH | The hybrid film did not promote changes in the quality parameters. | [48] |
| Chitosan (10% w/v) | Commercial Size: 30 nm | Gelatin (4% w/v), glycerol (25%) | Evaporative casting/coating | White cheese | 4 °C for 12 days at 80% RH | The hybrid coating protected the physical and chemical quality, reduced the weight loss and inhibited bacterial growth. | [48] |
| Chitosan (3% w/v) | Conc. 3% | Roselle calyx extracts (2.8 g) guar gum (3% w/v) | Evaporative casting/coating | Ras cheese | 12 °C for three months at 80% RH | Nanocomposite films enhanced the shelf life of cheese without changes in its sensorial properties. | [13] |
| Chitosan (2% w/v) | Conc.: 2–8% Commercial | CMC (1% w/v) | Evaporative casting/coating | White cheese from buffalo milk | 7 °C for 30 days | Cheese was microbiological stable during storage. | [49] |
| CMC (1% w/v) | Commercial | Cinnamaldehyde (100 mg/100 mL) | Evaporative casting/coating | Cherry tomato | 25 °C for 10 days at 45% RH | Nanocomposite film reduced changes in weight and firmness. | [50] |
| CMC (0.5% w/v) | Conc.: 0.2% (w/v) Commercial Size: 30–100 nm | NR | Evaporative casting/coating | Pomegranate | 4 °C for 12 days at 90% RH | The composite delayed the fruit ripening process. | [51] |
| Buckwheat starch (30 g/L) | Conc.: 3% Commercial Size: <50 nm | Sorbitol (15 g/L) | Evaporative casting/coating | Mushrooms | 4 °C for 6 days | Treated mushrooms exhibited reduced dehydration. | [52] |
| Sodium alginate (1.5% w/v) | Conc.: 1.25 g/L Commercial Size: 30–50 nm | Glycerol (1%) | Dipping/film | Strawberries | 1 °C for 20 days at 95% RH | The hybrid films reduced microbial infections and activated the antioxidant system of the fruit. | [53] |
| Sodium alginate (1.67 g in 50 mL) | Conc.: 1 mg/mL Size: 5 to 10 nm | Calcium chloride (5% w/w) Glycerol (1.5 mL) | Evaporative casting/coating | Smoked salmon | 4 °C for 4 days | Treated salmon was microbiologically stable during storage. | [42] |
| Calcium alginate (0.7 g in 30 mL) | Conc.: 3 mg/mL Size: 50 nm | NI | Evaporative casting/coating | Poultry meat | 4 °C for 10 days | Hybrid coating provide microbial control during storage. | [54] |
| Carrageenan (8 × 10−4 kg) | Conc.: 1% w/v Commercial Size: 20 × 10−9 m | Glycerol (5 × 10−4 L) | Evaporative casting/film | Mango | 20 °C at 60% RH | Treated fruit retained firmness and retarded the ripening process. | [55] |
| Pectin (10 g) | Concentration (100 mg/L) Size: 43.1 nm | Glycerol (1 mL) | Dipping/film | Starfruit | 8 days | Treated fruit showed minimal moldy infections and preserved quality attributes during storage. | [56] |
| Agar | Conc.: 1% | Glycerol (1% v/v) | Evaporative casting/Coating | Smoked salmon | 4 °C for 8 days | Hybrid coating provide microbial control and reduced lipid oxidation during storage. | [57] |
| Polysaccharide | TiO2 Specifications | Other Additives | Coating Method/Presentation | Food Product | Storage Conditions | Observed Results | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan (1% w/w, DD 85%) | Conc.: 1% w/w Commercial Size: 15 nm | Thymol (0.5%); tween-80 (0.25%) Acetic acid (1 mL/100 mL) | Dipping for 1 min and air-dried at 25 °C/film | Cantaloupe fruit | 25 °C for 8 days | Treated fruit showed microbial safety and maintain quality parameters during storage. | [63] |
| Chitosan (1% w/w, DD >95%) | Conc.: 0.3% w/w Commercial Size: 30 nm | Glycerol (1% w/w) Acetic acid (1 mL/100 mL) | Dipping for 3 min and air-dried/film | Mango fruit | 13 °C for 20 days | The hybrid film preserved the quality parameters of mangoes. | [64] |
| Chitosan (1% w/w, DD >99%) | Commercial | Graphene oxide (1 mg/mL) Acetic acid (0.5 mL/100 mL) Glutaraldehyde solution (2 mL) | NI/NI | Mangoes | 25 °C for 14 days | Coated fruit maintained their color attributes. | [65] |
| Chitosan (1% w/w, DD >99%) | Commercial | Graphene oxide (1 mg/mL) Acetic acid (0.5 mL/100 mL) Glutaraldehyde solution (2 mL) | NI/NI | Strawberries | 25 °C for 14 days | Coated fruit maintained their color attributes. | [65] |
| Chitosan (2% w/w, DD >85%) | Conc.: 1% w/w Commercial Size: 21 nm | Glycerol (30% w/w of chitosan) Acetic acid (1 mL/100 mL) | Dipping/film | Tomatoes | 20 °C for 15 days | Treated fruit showed minimal changes in quality parameters and delayed the ripening process. | [11] |
| Chitosan (1% w/w, DD 90%) | Conc.: 0.05 g Size: 50–80 nm | Acetic acid (2.5% v/v) | Dipping/film | Red grapefruit | 37 °C for 22 days | Hybrid film prevented microbial infection and extended the shelf life of fruit. | [62] |
| Chitosan (1% w/w, DD >90%) | Conc.: 0.03% w/w Anatase phase Size: <200 nm | Glycerol (6.5% v/v) Acetic acid (1 mL/100 mL) | NI/film | Stauntonvine fruit | 25 °C for 45 days | Fruit treated with hybrid film showed good CO2 transmission without significant changes in quality parameters. | [66] |
| Chitosan (1% w/w, DD 85%) | Conc.: 1% w/w Size: 15 nm | Thymol (0.5%); tween-80 (0.25%) Acetic acid (1 mL/100 mL) | NI/film | Mushroom | 4 °C for 12 days | Hybrid films reduced the PPO activity and inhibited the microbial pollution growth. | [67] |
| Chitosan (1% w/w, DD >75%) | Conc.: 0.02% w/v Size: 18 nm Commercial | NI | Dipping for 1 min and air-dried/film | Ginko biloba seeds | 1 °C for 180 days | Hybrid films prevented mildew apparition. | [68] |
| Chitosan (2% w/w) | Conc.: 1% w/w Commercial | Tween-80 (0.25%) Glycerol (0.75 mL/g chitosan) Acetic acid (1 mL/100 mL) CCEO (1.5% v/v) | Evaporative casting/coating | Minced meat | 4 °C for 7 days | Meat was microbially stable during storage. | [69] |
| Cellulose (1% w/v) | Conc.: 1% w/w Commercial Size: 10–25 nm Phase: anatase | WPI (10% w/v) Glycerol (6% w/v) REO (2% w/v) | Evaporative casting at 30 °C/coating | Lamb meat | 4 °C for 15 days | Meat was microbially stable during storage. | [70] |
| Cellulose (1% w/v) | Conc.: 1% w/w Commercial Size: 10–25 nm Phase: anatase | WPI (10% w/v) Glycerol (6% w/v) REO (2% w/v) | Evaporative casting at 30 °C/coating | Lamb meat | 4 °C for 15 days | Hybrid films reduced lipid peroxidation. | [71] |
| Starch | 0.01% w/w | Glycerol Distilled vinegar (5%) | Evaporative casting at 35 °C/coating | Bananas | Ambient temp. for 14 days | Hybrid films extended the shelf life compared to uncoated fruit. | [37] |
| Starch | 0.01% w/w | Glycerol Distilled vinegar (5%) | Evaporative casting at 35 °C/coating | Tomatoes | Ambient temp. for 21 days | Hybrid films extended the shelf life compared to uncoated fruit. | [37] |
| Guar gum | NI | NI | Dipping for 1 min and air-dried/film | Dates | 0 °C for 60 days | Treated fruit preserved quality parameters during storage. | [72] |
| Polysaccharide | Ag Specifications | Other Additives | Coating Method/Presentation | Food Product | Storage Conditions | Observed Results | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan (1% w/w) | Commercial Size: 200 nm | Phosphatidylcholine and cholesterol (50 mg at molar ratio of 5:1) | Evaporative casting/films | Pork meat | 4 °C for 15 days | Hybrid films preserved the meat quality during. | [77] |
| Bacterial cellulose Piece (2 cm × 2.5 cm × 0.3 cm) | Conc.: 1% w/w Size: 10 nm | NI | Evaporative casting/plasmonic nanopaper | Fish | 60 h | Color change suggested a food decomposition process. | [78] |
| Bacterial cellulose Piece (2 cm × 2.5 cm × 0.3 cm) | Conc.: 1% w/w Size: 10 nm | NI | Evaporative casting/plasmonic nanopaper | Meat | 60 h | Color change suggested a food decomposition process. | [78] |
| Cellulose acetate (1% w/w) | Conc.: 0.05 g Size: ~7 to 40 nm | Triethyl citrate (0.2 g) thymol (0.08 g) | Evaporative casting/films | Ethanol (as fatty food simulant) | NI | Hybrid films exhibited antioxidant properties. | [79] |
| Sodium alginate (10% w/w) | Conc.: 80 µg/mL Size: Size of 5–40 nm | Glycerol (1 mL) | Evaporative casting/films | Carrots | 4 °C for 10 days | Hybrid films enhanced the shelf life of carrots. | [16] |
| Sodium alginate (10% w/w) | Conc.: 80 µg/mL Size: Size of 5–40 nm | Glycerol (1 mL) | Evaporative casting/films | Pears | 4 °C for 10 days | Hybrid films enhanced the shelf life of pears. | [16] |
| Polysaccharide | SiO2 Specifications | Other Additives | Coating Method/Presentation | Food Product | Storage Conditions | Observed Results | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan (2% w/w) | NI | PVA (1% w/w) | Evaporative casting/film | Cherries | NI | Hybrid film prevented loss weight and enzymatic browning. | [86] |
| Chitosan (1% w/w, DD 85%) | Size: 15 nm | Nisin (1% w/w) Glycerol (0.5%) Acetic acid (1 mL/100 mL) | NI/film | Blueberries | 28 °C for 8 days | Hybrid films prevented fruit decay and preserved their quality parameters. | [87] |
| Potato starch (5% w/w) | Conc.: 0.3% w/w Commercial | Glycerin (5% w/w) | Evaporative casting/ film | White mushroom | 4 °C for 12 days | Hybrid films did not promote changes in the quality parameters during storage. | [85] |
| Hydroxy propyl methyl cellulose (4% w/w) | Conc.: 80 ppm Size: ~80 nm | Glycerol at 30% w/w | Evaporative casting/coating | Chicken fillets | 4 °C for 15 days | Hybrid films prevented microbial infection of foodborne pathogens. | [15] |
| Nanoparticles Specifications | Polysaccharide | Other Additives | Coating Method/Presentation | Food Product | Storage Conditions | Observed Results | Ref. |
|---|---|---|---|---|---|---|---|
| Halloysite Conc.: 6 g Commercial | Starch (25% w/w) | Glycerol (25 g) Nisin (6 g) | Extrusion/film | Minas Frescal cheese | 4 °C for 14 days | Inhibited Listeria monocytogenes proliferation. | [14] |
| Aluminum oxide Conc.: 80 ppm Size of ~80 nm | Hydroxy propyl methyl cellulose (4% w/w) | Glycerol (30% w/w) | Evaporative casting/film | Chicken fillets | 4 °C for 15 days | Coated meat was microbially stable during storage. | [15] |
| Montmorillonite Conc.: 2.5% w/w Commercial | Chitosan (1.5% w/w) | Glacial acid (1% v/v) glycerol (30% w/w), REO or GEO (2% v/v) Tween 80 (0.2% w/v) | Evaporative casting/film | Poultry meat | 5 °C for 15 days | Hybrid films preserved the quality parameters during storage. | [88] |
| Iron(III) oxide | Sodium alginate | NI | NI/coating | Apples, carrots, and brinjal | 25 °C | Hybrid films retarded decay in coated products. | [89] |
| Iron(III) oxide | Cellulose | NI | NI/coating | Apples, carrots, and brinjal | 25 °C | Hybrid films retarded decay in coated products. | [89] |
| Zirconium Conc.: 4 mmol Commercial | Chitosan (3% w/v) | Glacial acid (3% v/v) | Co-precipitation method/film | Tomatoes | 25 °C for 7 days | Hybrid films prevented fungal infection. | [90] |
| Magnesium oxide Conc.: 0.2% w/w Commercial Size of 20 nm | Chitosan (2% w/w) | BSM (2% w/w), glycerol (0.75% w/w) ZEO (2% w/w), Tween 80 (0.25% w/v) | Evaporative casting/coating | Rainbow trout fillets | 4 °C for 18 days | Coated fillets showed extended shelf life without changes in sensory attributes. | [91] |
| Magnesium oxide Conc.: 0.05% w/v Commercial Size of 20 nm | Cellulose (5% v/v) | Gelatin (20%, w/v) glycerin (3%, v/v) | Evaporative casting/coating | Processed Eggs | 25 °C for 112 days | Hybrid films extended the food shelf life. | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Esparza, L.M.; Villagrán-de la Mora, Z.; Rodríguez-Barajas, N.; Ruvalcaba-Gómez, J.M.; Iñiguez-Muñoz, L.E.; Maytorena-Verdugo, C.I.; Montalvo-González, E.; Pérez-Larios, A. Polysaccharide-Based Packaging Functionalized with Inorganic Nanoparticles for Food Preservation. Polysaccharides 2021, 2, 400-428. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2020026
Anaya-Esparza LM, Villagrán-de la Mora Z, Rodríguez-Barajas N, Ruvalcaba-Gómez JM, Iñiguez-Muñoz LE, Maytorena-Verdugo CI, Montalvo-González E, Pérez-Larios A. Polysaccharide-Based Packaging Functionalized with Inorganic Nanoparticles for Food Preservation. Polysaccharides. 2021; 2(2):400-428. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2020026
Chicago/Turabian StyleAnaya-Esparza, Luis Miguel, Zuamí Villagrán-de la Mora, Noé Rodríguez-Barajas, José Martín Ruvalcaba-Gómez, Laura Elena Iñiguez-Muñoz, Claudia Ivette Maytorena-Verdugo, Efigenia Montalvo-González, and Alejandro Pérez-Larios. 2021. "Polysaccharide-Based Packaging Functionalized with Inorganic Nanoparticles for Food Preservation" Polysaccharides 2, no. 2: 400-428. https://0-doi-org.brum.beds.ac.uk/10.3390/polysaccharides2020026