Wastewater Treatment from Lead and Strontium by Potassium Polytitanates: Kinetic Analysis and Adsorption Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of PPT Sorbent
2.2. Sorption Capacity Assessment
2.3. Numerical Analysis of Adsorption Kinetics
3. Results and Discussion
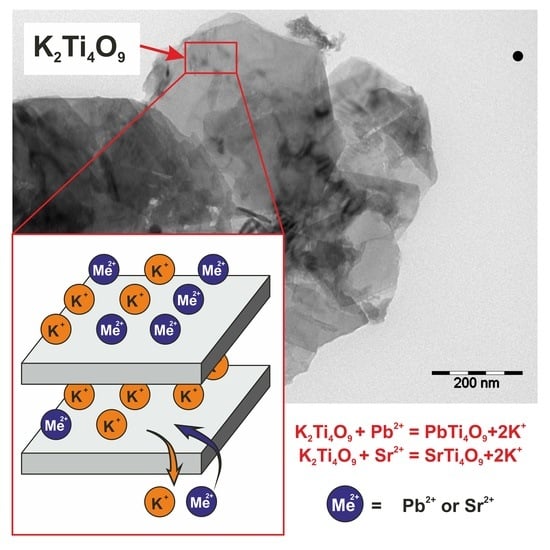
3.1. Characterization of Sorbents
3.2. Change of Sorption Rate
3.3. Analysis of the Diffusion Component of the Sorption Process (Boyd Model)
3.4. Analysis of Chemical Kinetics of Sorption Processes
3.5. PPT Sorption Capacity Compared to Published Sorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Alvarez-Ayuso, E.; Garcıa-Sánchez, A.; Querol, X. Purification of metal electroplating wastewaters using zeolites. Water Res. 2003, 37, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.Γ.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Chui, Z.C.; He, W.Q. Industrial Wastewater Treatment; Metallurgical Industry Press: Beijing, China, 1999. [Google Scholar]
- Hu, Q.H.; Weng, J.Q.; Wang, J.S. Sources of anthropogenic radionuclides in the environment: A review. J. Environ. Radioact. 2010, 101, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Guhathakurta, H.; Kaviraj, A. Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackish water ponds of Sunderban, India. Mar. Pollut. Bull. 2000, 40, 914–920. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661. [Google Scholar] [CrossRef] [PubMed]
- Jiuhui, Q.U. Research progress of novel adsorption processes in water purification: A review. J. Environ. Sci. 2008, 20, 1–13. [Google Scholar]
- Shahmirzadi, M.A.A.; Hosseini, S.S.; Luo, J.; Ortiz, I. Significance, evolution and recent advances in adsorption technology, materials and processes for desalination, water softening and salt removal. J. Environ. Manag. 2018, 215, 324–344. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Płaza, A.; Kołodyńska, D.; Hałas, P.; Gęca, M.; Franus, M.; Hubicki, Z. The zeolite modified by chitosan as an adsorbent for environmental applications. Adsorpt. Sci. Technol. 2017, 35, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, G.; Andal, N.M.; Anbalagan, K. Adsorption studies of iron (III) on chitin. J. Chem. Sci. 2005, 117, 663–672. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Anbalagan, K.; Andal, N.M. Adsorption dynamics and equilibrium studies of Zn (II) onto chitosan. J. Chem. Sci. 2004, 116, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. A review and experimental verification of using chitosan and its derivatives as adsorbents for selected heavy metals. J. Environ. Manag. 2010, 91, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Xue, C.; Qi, P.; Liu, Y. Adsorption of aquatic Cd2+ using a combination of bacteria and modified carbon fiber. Adsorpt. Sci. Technol. 2018, 36, 857–871. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Bulin, C.; Li, B.; Zhao, Z.; Yu, H.; Sun, H.; Ge, X.; Xing, R.; Zhang, B.; Zhang, B. Efficient removal of aqueous Pb(II) using partially reduced graphene oxide-Fe3O4. Adsorpt. Sci. Technol. 2018, 36, 1031–1048. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Ahalya, N.; Ramachandra, T.V.; Kanamadi, R.D. Biosorption of heavy metals. Res. J. Chem. Environ. 2008, 7, 71–79. [Google Scholar]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef]
- Mishra, S.P.; Singh, V.K.; Tiwari, D. Radiotracer technique in adsorption study: Part XVII. Removal Behaviour of Alkali Metal (K- and Li-) Titanates for Cd(II). Appl. Radiat. Isot. 1998, 49, 1467–1475. [Google Scholar] [CrossRef]
- Nunes, L.M.; Cardoso, V.A.; Airoldi, C. Layered titanates in alkaline, acidic and intercalated with 1,8-octyldiamine forms as ion-exchangers with divalent cobalt, nickel and copper cations. Mater. Res. Bull. 2006, 41, 1089–1096. [Google Scholar] [CrossRef]
- Cardoso, V.D.A.; de Souza, A.G.; Sartoratto, P.P.; Nunes, L.M. The ionic exchange process of cobalt, nickel and copper (II) in alkaline and acid-layered titanates. Colloids Surf. A 2004, 248, 145–149. [Google Scholar] [CrossRef]
- Bitonto, L.; Volpe, A.; Pagano, M.; Bagnuolo, G.; Mascolo, G.; La Parola, V.; Di Leo, P.; Pastore, C. Amorphous boron-doped sodium titanates hydrates: Efficient and reusable adsorbents for the removal of Pb2+ from water. J. Hazard. Mater. 2017, 324, 168–177. [Google Scholar] [CrossRef]
- Sanchez-Monjaras, T.; Gorokhovsky, A.; Escalante-Garcia, J.I. Molten salt synthesis and characterization of potassium polytitanate ceramic precursors with varied TiO2/K2O molar ratios. J. Am. Ceram. Soc. 2008, 91, 3058–3065. [Google Scholar] [CrossRef]
- Burmistrov, I.N.; Kuznetsov, D.V.; Yudin, A.G.; Muratov, D.S.; Milyaeva, S.I.; Kostitsyn, M.A.; Gorshenkov, M.V. Analysis of the Effect of Preparation Conditions for Potassium Polytitanates on Their Morphological Properties. Refract. Ind. Ceram. 2012, 52, 393–397. [Google Scholar] [CrossRef]
- Tretyachenko, E.V.; Gorokhovsky, A.V.; Yurkov, G.Y.; Fedorov, F.S.; Vikulova, M.A.; Kovaleva, D.S.; Orozaliev, E.E. Adsorption and photo-catalytic properties of layered lepidocrocite-like quasi-amorphous compounds based on modified potassium polytitanates. Particuology 2014, 17, 22–28. [Google Scholar] [CrossRef]
- Gorokhovsky, A.V.; Tret’yachenko, E.V.; Vikulova, M.A.; Kovaleva, D.S.; Yurkov, G.Y. Effect of Chemical Composition on the Photocatalytic Activity of Potassium Polytitanates Intercalated with Nickel Ions. Russ. J. Appl. Chem. 2013, 86, 343–350. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Dyall, K.G. An exact separation of the spin-free and spin-dependent terms of the Dirac–Coulomb–Breit Hamiltonian. J. Chem. Phys. 1994, 100, 2118–2127. [Google Scholar] [CrossRef]
- Visscher, L.; Dyall, K.G. Dirac-Fock atomic electronic structure calculations using different nuclear charge distributions. At. Data Nucl. Data Tables 1997, 67, 207–224. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Absorption Gelöster Stoffe; P. A. Norstedt & Söner: Stockholm, Sweden, 1898. [Google Scholar]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Rev. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Biryukova, M.I.; Burmistrov, I.N.; Yurkov, G.Y.; Mazov, I.N.; Ashmarin, A.A.; Gorokhovskii, A.V.; Gryaznov, V.I.; Buznik, V.M. Development of a Fibrous Potassium Polytitanate. Theor. Found. Chem. Eng. 2015, 49, 485–489. [Google Scholar] [CrossRef]
- Rabinovich, V.A.; Havin, Z.Y. Handbook of Chemistry; Khimiya: Leningrad, Russia, 1991; p. 432. [Google Scholar]
- Zanello, P. Inorganic Electrochemistry: Theory, Practice and Application; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Ivanov, V.M.; Figurovskaya, V.N.; Burmaa, D. Photometric determination of cobalt and erbium in their binary alloys. Vestn. MGU Ser. 2 Khimiya 1999, 40, 98–102. [Google Scholar]
- Tao, Y.; Ye, L.; Pan, J.; Wang, Y.; Tang, B. Removal of Pb(II) from aqueous solution on chitosan/TiO2 hybrid film. J. Hazard. Mater. 2009, 161, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, L.; Chen, Y.; Fang, M.; Zhang, J.; Wang, H. Highly efficient, irreversible and selective ion exchange property of layered titanate nanostructures. Adv. Funct. Mater. 2012, 22, 835–841. [Google Scholar] [CrossRef]
- Huang, J.; Cao, Y.; Liu, Z.; Deng, Z.; Tang, F.; Wang, W. Efficient removal of heavy metal ions from water system by titanate nanoflowers. Chem. Eng. J. 2012, 180, 75–80. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chen, H.; Jiang, F. Adsorption of Pb(II) Using Magnetic Titanate Nanotubes Prepared via Two-Step Hydrothermal Method. CLEAN–Soil Air Water 2014, 42, 947–955. [Google Scholar] [CrossRef]
- Yang, D.; Zheng, Z.; Liu, H.; Zhu, H.; Ke, X.; Xu, Y.; Wu, D.; Sun, Y. Layered titanate nanofibers as efficient adsorbents for removal of toxic radioactive and heavy metal ions from water. J. Phys. Chem. C 2008, 112, 16275–16280. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Borthwick, A.G.; Wang, Y.; Yin, X.; Li, X.; Ni, J. Adsorption of Pb2+, Cd2+, Cu2+ and Cr3+ onto titanate nanotubes: Competition and effect of inorganic ions. Sci. Total Environ. 2013, 456, 171–180. [Google Scholar] [CrossRef]
- Wang, T.; Liu, W.; Xiong, L.; Xu, N.; Ni, J. Influence of pH, ionic strength and humic acid on competitive adsorption of Pb (II), Cd (II) and Cr (III) onto titanate nanotubes. Chem. Eng. J. 2013, 215, 366–374. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lo, S.L.; Kuo, J. Pb (II) adsorption capacity and behavior of titanate nanotubes made by microwave hydrothermal method. Colloids Surf. A 2010, 361, 126–131. [Google Scholar] [CrossRef]
- Xiong, L.; Chen, C.; Chen, Q.; Ni, J. Adsorption of Pb (II) and Cd (II) from aqueous solutions using titanate nanotubes prepared via hydrothermal method. J. Hazard. Mater. 2011, 189, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kim, S.; Hong, H.-J.; Hong, J.; Kim, M.; Ryu, T.; Park, I.-S.; Chung, K.-S.; Jang, J.; Kim, B.-G. Strontium ion (Sr2+) separation from seawater by hydrothermally structured titanate nanotubes: Removal vs. Recovery. Chem. Eng. J. 2016, 304, 503–510. [Google Scholar] [CrossRef]
- Wen, T.; Zhao, Z.; Shen, C.; Li, J.; Tan, X.; Zeb, A.; Wang, X.; Xu, A.-W. Multifunctional flexible free-standing titanate nanobelt membranes as efficient sorbents for the removal of radioactive 90Sr2+ and 137Cs+ ions and oils. Sci. Rep. 2016, 6, 20920. [Google Scholar] [CrossRef] [PubMed]







| Complex | Cation | Calculated Distance “Cation-Oxygen”, nm | Reviewed Distance “Cation-Oxygen” [35], nm | Divergence with Literary Values, % |
|---|---|---|---|---|
| K4Ti8O18 | K+ | 0.280 | 0.269 | 4 |
| Pb2Ti8O18 | Pb2+ | 0.232 | 0.262 | 11 |
| Sr2Ti8O18 | Sr2+ | 0.261 | 0.256 | 2 |
| Ions | Rate Constants | PPT Sorption Capacity for Pseudo-Second-Order Model | |
|---|---|---|---|
| k, min−1 | k, g·mg−1·min−1 | ||
| Pseudo−First Order Model | Pseudo−Second Order Model | ||
| Pb2+ | 0.0023 | 9.6·10−7 | 714.3 |
| Sr2+ | 0.0009 | 10·10−6 | 344.8 |
| Adsorbent | Ion | Adsorption Capacity, mg/g | Equilibrium Time, min | Adsorption Conditions | Reference |
|---|---|---|---|---|---|
| PPT | Pb2+ | 714.3 | 45 | pH = 4.5–6.2 | This article |
| Sr2+ | 344.8 | PH = 8.5–10.8 | |||
| Chitosan/TiO2 hybrid adsorbent | Pb2+ | 36.8 | 60 °C pH = 3–4 | [38] | |
| Na2Ti3O7 | Pb2+ | 563.6 | 60–120 | - | [39] |
| Titanates with various morphology | Pb2+ | 105–304 | 90 | - | [40] |
| Magnetic titanium nanotubes | Pb2+ | 442.5 | 60 | pH = 5 | [41] |
| Titanate nanofibers | Pb2+ | 244–280 | - | pH = 6–7 | [42] |
| Sr2+ | 50–55 | - | |||
| Titanate nanotubes | Pb2+ | 2.6 | - | pH = 5 | [43] |
| 299.5 | - | [44] | |||
| Titanate nanotubes obtained by hydrothermal method | Pb2+ | up to 2000 | 30 | pH = 4 | [45] |
| 520.8 | 180 | pH = 5–6 | [46] | ||
| Sr2+ | 91.7 | 10 | - | [47] | |
| 98.7 | 30 | - | [48] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolenko, A.; Shevelev, A.; Vikulova, M.; Blagova, T.; Altukhov, S.; Gorokhovsky, A.; Godymchuk, A.; Burmistrov, I.; Offor, P.O. Wastewater Treatment from Lead and Strontium by Potassium Polytitanates: Kinetic Analysis and Adsorption Mechanism. Processes 2020, 8, 217. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8020217
Ermolenko A, Shevelev A, Vikulova M, Blagova T, Altukhov S, Gorokhovsky A, Godymchuk A, Burmistrov I, Offor PO. Wastewater Treatment from Lead and Strontium by Potassium Polytitanates: Kinetic Analysis and Adsorption Mechanism. Processes. 2020; 8(2):217. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8020217
Chicago/Turabian StyleErmolenko, Anna, Alexey Shevelev, Maria Vikulova, Tatyana Blagova, Sergey Altukhov, Alexander Gorokhovsky, Anna Godymchuk, Igor Burmistrov, and Peter Ogbuna Offor. 2020. "Wastewater Treatment from Lead and Strontium by Potassium Polytitanates: Kinetic Analysis and Adsorption Mechanism" Processes 8, no. 2: 217. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8020217