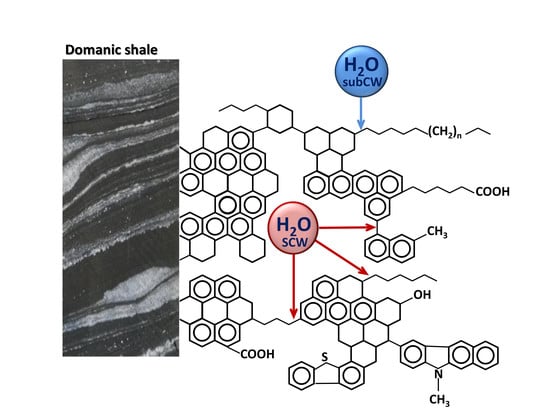
Heavy Oil Hydrocarbons and Kerogen Destruction of Carbonate–Siliceous Domanic Shale Rock in Sub- and Supercritical Water
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Zhai, G.; Zhang, G.; Wang, H.; Zhang, G.; Li, J.; Wang, Z.; Wen, Z.; Ma, F.; Liang, Y.; et al. Formation, distribution, potential and prediction of global conventional and unconventional hydrocarbon resources. Pet. Explor. Dev. 2015, 42, 14–28. [Google Scholar] [CrossRef]
- Chengzao, J.; Zheng, M.; Zhang, Y. Unconventional hydrocarbon resources in China and the prospect of exploration and development. Pet. Explor. Dev. 2012, 39, 139–146. [Google Scholar]
- Zou, C.N.; Zhu, R.K.; Wu, S.T.; Yang, Z.; Tao, S.Z.; Yuan, X.J.; Hou, L.H.; Yang, H.; Xu, C.C.; Li, D.H. Types, characteristics, genesis and prospects of conventional and unconventional hydrocarbon accumulations: Taking tight oil and tight gas in China as an instance. Acta Pet. Sin. 2012, 33, 173–187. [Google Scholar]
- Khisamov, R.S.; Bazarevskaya, V.G.; Tarasova, T.I.; Mikhailova, O.V.; Mikhailov, S.N. Geochemical evidence for petroleum potential of Domanic deposits in the Republic of Tatarstan (Russian). Oil Ind. J. 2016, 2016, 10–13. [Google Scholar]
- Khisamov, R.S.; Zakirov, I.S.; Zakharova, E.F.; Bazarevskaya, V.G.; Abusalimova, R.R.; Timirov, D.A. Experience of studying and development of domanic deposits on the example of bavlinskoye field of the republic of Tatarstan. Neft. Khozyaystvo Oil Ind. 2018, 11, 78–83. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhaylova, A.N.; Kosachev, I.P.; Morozov, V.P.; Vakhin, A.V. Gidrotermal’nyye prevrashcheniya organicheskogo veshchestva vysokouglerodistoy domanikovoy porody pri raznykh temperaturakh v uglekislotnoy srede. Neftekhimiya 2020, 60, 307–320. [Google Scholar]
- Bushnev, D.A.; Burdel’naya, N.S.; Shanina, S.N.; Makarova, E.S. Generation of hydrocarbons and hetero compounds by sulfur-rich oil shale in hydrous pyrolysis. Pet. Chem. 2004, 44, 416–425. [Google Scholar]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence, 2nd ed.; Springer: Berlin Heidelberg, Germany, 1984; p. 702. [Google Scholar] [CrossRef]
- Khisamov, R.S.; Bazarevskaya, V.G.; Yartiev, A.F.; Tarasova, T.I.; Gibadullina, O.G.; Mikhailova, O.V. Oil potential of Domanic productive formations in territory of Leninogorskneft activities. Neft. Khozyaystvo Oil Ind. 2015, 7, 10–14. [Google Scholar]
- Stoupakova, A.V.; Kalmykov, G.A.; Korobova, N.I.; Fadeeva, N.P.; Gatovskii, Y.A.; Suslova, A.A.; Sautkin, R.S.; Pronina, N.V.; Bolshakova, M.A.; Zavyalova, A.P.; et al. Domanic deposits of the Volga-Ural basin—Types of section, formation conditions and prospects of oil and gas potential. Georesursy 2017, 19, 112–124. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.M.; Feoktistov, D.A.; Morozov, V.P.; Vakhin, A.V. Conversion of the organic matter of domanic shale and permian bituminous rocks in hydrothermal catalytic processes. Energy Fuels 2017, 31, 7789–7799. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Onishchenko, Y.V.; Chemodanov, A.E.; Sitdikova, L.M.; Nurgaliev, D.K. Thermal transformation of bitumoid of Domanic formations of Tatarstan (Russian). Oil Ind. J. 2016, 2016, 32–34. [Google Scholar]
- Galimov, E.M.; Kamaleeva, A.I. Source of hydrocarbons in the supergiant Romashkino oilfield (Tatarstan): Recharge from the crystalline basement or source sediments? Geochemistry Int. 2015, 53, 95–112. [Google Scholar] [CrossRef]
- Whitehead, J.C.; Williams, D.F. Solvent extraction of coal by a supercritical gases. J. Inst. Fuel 1975, 48, 182. [Google Scholar]
- Funazukuri, T.; Yokoi, S.; Wakao, N. Supercritical fluid extraction of Chinese Maoming oil shale with water and toluene. Fuel 1988, 67, 10–14. [Google Scholar] [CrossRef]
- Yanik, J.; Yüksel, M.; Saǧlam, M.; Olukçu, N.; Bartle, K.; Frere, B. Characterization of the oil fractions of shale oil obtained by pyrolysis and supercritical water extraction. Fuel 1995, 74, 46–50. [Google Scholar] [CrossRef]
- Luik, L.; Luik, H.; Palu, V.; Kruusement, K.; Tamvelius, H. Conversion of the Estonian fossil and renewable feedstocks in the medium of supercritical water. J. Anal. Appl. Pyrolysis 2009, 85, 492–496. [Google Scholar] [CrossRef]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant: Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Siskin, M.; Katritzky, A.R. Review of the reactivity of organic compounds with oxygen-containing functionality in superheated water. J. Anal. Appl. Pyrolysis 2000, 54, 193–214. [Google Scholar] [CrossRef]
- Akiya, N.; Savage, P.E. Roles of water for chemical reactions in high-temperature water. Chem. Rev. 2002, 102, 2725–2750. [Google Scholar] [CrossRef]
- Al-alla, R.A.; Nassef, E. Extraction of Oil from Egyptian Oil Shale. J. Pet. Environ. Biotechnol. 2015, 6, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Abourriche, A.; Oumam, M.; Hannache, H.; Adil, A.; Pailler, R.; Naslain, R.; Birot, M.; Pillot, J.P. Effect of toluene proportion on the yield and composition of oils obtained by supercritical extraction of Moroccan oil shale. J. Supercrit. Fluids 2009, 51, 24–28. [Google Scholar] [CrossRef]
- Meng, M.; Hu, H.; Zhang, Q.; Ding, M. Extraction of tumuji oil sand with sub- and supercritical water. Energy Fuels 2006, 20, 1157–1160. [Google Scholar] [CrossRef]
- Arcelus-Arrillaga, P.; Pinilla, J.L.; Hellgardt, K.; Millan, M. Application of water in hydrothermal conditions for upgrading heavy oils: A review. Energy Fuels 2017, 31, 4571–4587. [Google Scholar] [CrossRef]
- Canıaz, R.O.; Arca, S.; Yaşar, M.; Erkey, C. Refinery bitumen and domestic unconventional heavy oil upgrading in supercritical water. J. Supercrit. Fluids 2019, 152, 1–10. [Google Scholar] [CrossRef]
- Daud, A.R.M.; Pinilla, J.L.; Arcelus-arrillaga, P.; Hellgardt, K.; Kandiyoti, R.; Millan, M. Heavy oil upgrading in subcritical and supercritical water: Studies on model compounds. Am. Chem. Soc. Div. Energy Fuels 2012, 57, 22. [Google Scholar]
- Gudiyella, S.; Lai, L.; Borne, I.H.; Tompsett, G.A.; Timko, M.T.; Choi, K.H.; Alabsi, M.H.; Green, W.H. An experimental and modeling study of vacuum residue upgrading in supercritical water. AIChE J. 2018, 64, 1732–1743. [Google Scholar] [CrossRef]
- Cheng, Z.-M.; Ding, Y.; Zhao, L.-Q.; Yuan, P.-Q.; Yuan, W.-K. Effects of supercritical water in vacuum residue upgrading. Energy Fuels 2009, 23, 3178–3183. [Google Scholar] [CrossRef]
- Canel, M.; Missal, P. Extraction of solid fuels with sub-and supercritical water. Fuel 1994, 73, 1776–1780. [Google Scholar] [CrossRef]
- Olukcu, N.; Yanik, J.; Saglam, M.; Yuksel, M.; Karaduman, M. Solvent effect on the extraction of Beypazari oil shale. Energy Fuels 1999, 13, 895–902. [Google Scholar] [CrossRef]
- Nasyrova, Z.R.; Kayukova, G.P.; Onishchenko, Y.V.; Morozov, V.P.; Vakhin, A.V. Conversion of high-carbon Domanic Shale in sub-and supercritical water. Energy Fuels 2020, 34, 1329–1336. [Google Scholar] [CrossRef]
- Nasyrova, Z.R.; Kayukova, G.P.; Khasanova, N.M.; Vakhin, A.V. Transformation of organic matter of domanik rock from the Romashkino oilfield in sub- and supercritical water. Pet. Chem. 2020, 60. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G.; Stein, S.E. NIST Chemistry WebBook (NIST Standard Reference Database vol 69). 2017. Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 March 2020).
- Lopatin, N.V.; Emets, T.P. Pyrolysis in Oil and Gas Geochemistry (in Russian); Nauka: Moscow, Russia, 1987. [Google Scholar]
- Han, L.; Zhang, R.; Bi, J. Experimental investigation of high-temperature coal tar upgrading in supercritical water. Fuel Process. Technol. 2009, 90, 292–300. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Kiyamova, A.M.; Mikhailova, A.N.; Kosachev, I.P.; Petrov, S.M.; Romanov, G.V.; Sitdikova, L.M.; Plotnikova, I.N.; Vakhin, A.V. Generation of hydrocarbons by hydrothermal transformation of organic matter of Domanik rocks. Chem. Technol. Fuels Oils 2016, 52, 149–161. [Google Scholar] [CrossRef]
- Antipenko, V.R.; Bakanova, O.S.; Kashapov, R.S. Kharakteristika termicheskoy ustoychivosti masel prirodnykh bitumov i neftey. Izv. Tomsk. Politekh. Univ. Inzhiniring Georesursov. 2019, 330, 152–160. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Eskin, A.A.; Morozov, V.I. Effect of the natural minerals pyrite and hematite on the transformation of Domanik rock organic matter in hydrothermal processes. Pet. Chem. 2019, 59, 24–33. [Google Scholar] [CrossRef]
- Fedyaeva, O.N.; Antipenko, V.R.; Dubov, D.Y.; Kruglyakova, T.V.; Vostrikov, A.A. Non-isothermal conversion of the Kashpir sulfur-rich oil shale in a supercritical water flow. J. Supercrit. Fluids 2016, 109, 157–165. [Google Scholar] [CrossRef]
- Peter, K.E.; Walter, C.C.; Moldowan, M. The Biomarker Guide, Volume 2—Biomarkers and Isotopes in Petroleum Exploration and Earth History; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bennett, B.; Adams, J.J.; Larter, S.R. Oil fingerprinting for production allocation: Exploiting the natural variations in fluid properties encountered in heavy oil and oil sand reservoirs. In Proceedings of the Frontiers + Innovation – 2009 CSPG CSEG CWLS Convention, Calgary, AB, Canada, 4–8 May 2009; pp. 157–160. [Google Scholar]
- Jameel, A.G.A.; Han, Y.; Brignoli, O.; Telalović, S.; Elbaz, A.M.; Im, H.G.; Roberts, W.L.; Sarathy, S.M. Heavy fuel oil pyrolysis and combustion: Kinetics and evolved gases investigated by TGA-FTIR. J. Anal. Appl. Pyrolysis 2017, 127, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Zhang, C.; Yang, F.; Heng, M.; Shao, P.; Wang, Y. Influence of temperature field on rock and heavy components variation during in-situ combustion process. Fuel 2018, 230, 244–257. [Google Scholar] [CrossRef]
- Ganz, H.; Kalkreuth, W. Application of infrared spectroscopy to the classification of kerogentypes and the evaluation of source rock and oil shale potentials. Fuel 1987, 66, 708–711. [Google Scholar] [CrossRef]
- Craddock, P.R.; Le Doan, T.V.; Bake, K.; Polyakov, M.; Charsky, A.M.; Pomerantz, A.E. Evolution of kerogen and bitumen during thermal maturation via semi-open pyrolysis investigated by infrared spectroscopy. Energy Fuels 2015, 29, 2197–2210. [Google Scholar] [CrossRef]
- Iglesias, M.J.; Jimenez, A.; Laggoun-Défarge, F.; Suarez-Ruiz, I. FTIR study of pure vitrains and associated coals. Energy Fuels 1995, 9, 458–466. [Google Scholar] [CrossRef]
- Lin, R.; Ritz, G.P. Studying individual macerals using ir microspectrometry, and implications on oil versus gas/condensate proneness and “low-rank” generation. Org. Geochem. 1993, 20, 695–706. [Google Scholar] [CrossRef]
- Lis, G.P.; Mastalerz, M.; Schimmelmann, A.; Lewan, M.D.; Stankiewicz, B.A. FTIR absorption indices for thermal maturity in comparison with vitrinite reflectance R0 in type-II kerogens from Devonian black shales. Org. Geochem. 2005, 36, 1533–1552. [Google Scholar] [CrossRef]
- Geng, W.; Nakajima, T.; Takanashi, H.; Ohki, A. Analysis of carboxyl group in coal and coal aromaticity by Fourier transform infrared (FT-IR) spectrometry. Fuel 2009, 88, 139–144. [Google Scholar] [CrossRef]
- Ibarra, J.; Munoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Bushnev, D.A.; Burdel’naya, N.S. Khimicheskaya struktura kerogena i usloviya yego formirovaniya. Geol. Geofiz. 2009, 50, 822–829. [Google Scholar]
- Kawamura, K.; Tannenbaum, E.; Huizinga, B.J.; Kaplan, I.R. Long-chain carboxylic acids in pyrolysates of Green River kerogen. Org. Geochem. 1986, 10, 1059–1065. [Google Scholar] [CrossRef]
- Punanova, S.A. Mikroelementy Neftej, Ich Ispol’zovanie pri Geochimičeskich Issledovanijach i Izucenii Processov Migracii; Nedra: Moscow, Russia, 1974. [Google Scholar]
- Xu, J.B.; Cheng, B.; Deng, Q.; Liang, Y.G.; Faboya, O.L.; Liao, Z.W. Distribution and geochemical significance of trace elements in shale rocks and their residual kerogens. Acta Geochim. 2018, 37, 886–900. [Google Scholar] [CrossRef]
- Gottikh, R.P.; Pisotsky, B.I.; Plotnikova, I.N. Informativity of trace elements in the oil geology. Georesursy 2012, 47, 24–31. [Google Scholar]









| Object | Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Corg | H/Corg | Tmax | S1 | S2 | GP | PI | HI | |
| Initial | 7.07 | 2.87 | 429 | 1.52 | 22.17 | 23.69 | 0.06 | 313.58 |
| * | 4.06 | 2.85 | 432 | 0.27 | 17.84 | 18.11 | 0.01 | 439.41 |
| Sub-CW | 6.98 | 2.18 | 432 | 1.06 | 17.79 | 18.85 | 0.06 | 254.87 |
| * | 4.02 | 1.28 | 434 | 0,27 | 17.01 | 17.28 | 0.02 | 302.13 |
| SCW | 4.08 | 5.44 | 435 | 1.79 | 1.95 | 3.74 | 0.48 | 47.79 |
| * | 3.12 | 5.50 | 433 | 0.39 | 2.42 | 2.82 | 0.14 | 77.56 |
| Parameters | Object | ||
|---|---|---|---|
| Initial | Sub-CW | SCW | |
| Temperature of experiment, °C | - | 320 | 374 |
| Pressure of experiment, MPa | - | 17.0 | 24.6 |
| Gas yield, %wt. | - | 2.26 | 2.65 |
| Heavy oil yield, %wt. | 3.12 | 3.98 | 3.03 |
| Saturated, %wt. | 14.81 | 16.89 | 33.91 |
| Aromatics, %wt. | 19.17 | 22.74 | 14.33 |
| Resins, %wt. | 37.00 | 27.46 | 13.49 |
| Asphaltenes, %wt. | 29.02 | 32.91 | 23.78 |
| Carbene–carboids, %wt. | - | - | 14.49 |
| Object | Gases Composition *, % vol. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 | O2 | CO2 | CH4 | C2H4 | C2H6 | C3H6 | C3H8 | C4H8 | n-C4H10 | |
| Sub-CW | 0.23 | 10.89 | 83.76 | 3.96 | 0.00 | 0.00 | 0.12 | 0.02 | 0.08 | 0.25 |
| SCW | 0.51 | 9.98 | 64.60 | 11.66 | 0.12 | 5.96 | 0.00 | 3.93 | 0.36 | 0.99 |
| Object | Structural Parameters | ||||
|---|---|---|---|---|---|
| CH3/CH2 Ratio | A-Factor | C-Factor | Aromaticity | Degree of Condensation | |
| Saturated hydrocarbons | |||||
| Initial | 0.04 | 0.55 | 0.99 | 0.34 | 1.17 |
| Sub-CW | 0.04 | 0.53 | 0.99 | 0.37 | 1.14 |
| SCW | 0.04 | 0.54 | 0.97 | 0.24 | 1.45 |
| Aromatic hydrocarbons | |||||
| Initial | 0.62 | 0.95 | 0.34 | 1.19 | 0.88 |
| Sub-CW | 0.57 | 0.95 | 0.42 | 1.21 | 0.83 |
| SCW | 0.63 | 0.89 | 0.23 | 1.15 | 0.55 |
| Resins | |||||
| Initial | 0.70 | 0.86 | 0.39 | 3.28 | 0.94 |
| Sub-CW | 0.61 | 0.84 | 0.42 | 1.09 | 0.38 |
| SCW | 0.71 | 0.71 | 0.44 | 2.89 | 0.40 |
| Asphaltenes | |||||
| Initial | 0.60 | 0.74 | 0.21 | 0.46 | 0.19 |
| Sub-CW | 0.62 | 0.71 | 0.17 | 0.65 | 0.24 |
| SCW | 0.67 | 0.62 | 0.16 | 1.26 | 0.27 |
| Carbene–carboids | |||||
| SCW | 0.99 | 0.28 | 0.09 | 3.16 | 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasyrova, Z.R.; Kayukova, G.P.; Vakhin, A.V.; Djimasbe, R.; Chemodanov, A.E. Heavy Oil Hydrocarbons and Kerogen Destruction of Carbonate–Siliceous Domanic Shale Rock in Sub- and Supercritical Water. Processes 2020, 8, 800. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070800
Nasyrova ZR, Kayukova GP, Vakhin AV, Djimasbe R, Chemodanov AE. Heavy Oil Hydrocarbons and Kerogen Destruction of Carbonate–Siliceous Domanic Shale Rock in Sub- and Supercritical Water. Processes. 2020; 8(7):800. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070800
Chicago/Turabian StyleNasyrova, Zukhra R., Galina P. Kayukova, Alexey V. Vakhin, Richard Djimasbe, and Artem E. Chemodanov. 2020. "Heavy Oil Hydrocarbons and Kerogen Destruction of Carbonate–Siliceous Domanic Shale Rock in Sub- and Supercritical Water" Processes 8, no. 7: 800. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070800