Identification of Copper in Stems and Roots of Jatropha curcas L. by Hyperspectral Imaging
Abstract
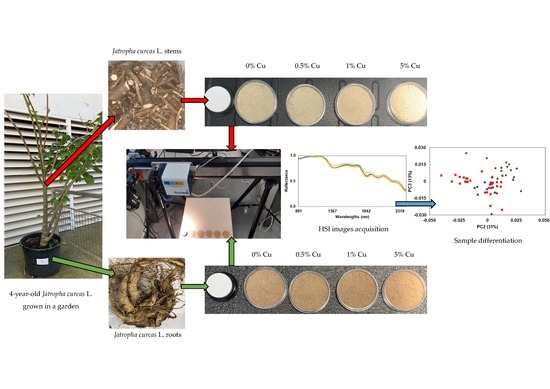
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Metal Content Analysis
2.3. Sample Preparation and Image Acquisition
2.4. Spectra Extraction and Multivariate Analysis
2.5. Principal Components Analysis (PCA)
2.6. Linear Discriminant Analysis (LDA)
3. Results and Discussion
3.1. Spectral Analysis
3.2. Principal Components Analysis (PCA)
3.3. Linear Discriminant Analysis (LDA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Moraza, C.; Iglesias, N.; Palencia, I. Application of sugar foam to a pyrite-contaminated soil. Miner. Eng. 2006, 19, 399–406. [Google Scholar] [CrossRef]
- Shi, T.; Chen, Y.; Liu, Y.; Wu, G. Visible and near-infrared reflectance spectroscopy-An alternative for monitoring soil contamination by heavy metals. J. Hazard. Mater. 2014, 265, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Mateos, P.; Alés-Álvarez, F.J.; García-Martín, J.F. Phytoremediation of highly contaminated mining soils by Jatropha curcas L. and production of catalytic carbons from the generated biomass. J. Environ. Manag. 2019, 231, 886–895. [Google Scholar] [CrossRef] [PubMed]
- García Martín, J.F.; del González Caro, M.C.; del López Barrera, M.C.; Torres García, M.; Barbin, D.; Álvarez-Mateos, P. Metal accumulation by Jatropha curcas L. adult plants grown on heavy metal-contaminated soil. Plants 2020, 9, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badaró, A.T.; Garcia-Martin, J.F.; del López-Barrera, M.C.; Barbin, D.F.; Alvarez-Mateos, P. Determination of pectin content in orange peels by near infrared hyperspectral imaging. Food Chem. 2020, 323, 126861. [Google Scholar] [CrossRef] [PubMed]
- García Martín, J.F. Optical path length and wavelength selection using Vis/NIR spectroscopy for olive oil’s free acidity determination. Int. J. Food Sci. Technol. 2015, 50, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Lopes, J.F.; Ludwig, L.; Barbin, D.F.; Grossmann, M.V.E.; Barbon, S. Computer vision classification of barley flour based on spatial pyramid partition ensemble. Sensors 2019, 19, 2953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of near-infrared (NIR) spectroscopy and hyperspectral imaging for quality and safety assessment of fruits: An overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Moros, J.; De Vallejuelo, S.F.O.; Gredilla, A.; De Diego, A.; Madariaga, J.M.; Garrigues, S.; De La Guardia, M. Use of reflectance infrared spectroscopy for monitoring the metal content of the estuarine sediments of the Nerbioi-Ibaizabal River (Metropolitan Bilbao, Bay of Biscay, Basque Country). Environ. Sci. Technol. 2009, 43, 9314–9320. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.; Ji, J.; Gong, P.; Liao, Q.; Tian, Q.; Ma, H. A Mechanism study of reflectance spectroscopy for investigating heavy metals in soils. Soil Sci. Soc. Am. J. 2007, 71, 918–926. [Google Scholar] [CrossRef]
- Rathod, P.H.; Rossiter, D.G.; Noomen, M.F.; van der Meer, F.D. Proximal spectral sensing to monitor phytoremediation of metal-contaminated soils. Int. J. Phytoremediat. 2013, 15, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Manios, T.; Stentiford, E.I.; Millner, P.A. The effect of heavy metals accumulation on the chlorophyll concentration of Typha latifolia plants, growing in a substrate containing sewage sludge compost and watered with metaliferus water. Ecol. Eng. 2003, 20, 65–74. [Google Scholar] [CrossRef]
- Verdú, S.; Vásquez, F.; Ivorra, E.; Sánchez, A.J.; Barat, J.M.; Grau, R. Hyperspectral image control of the heat-treatment process of oat flour to model composite bread properties. J. Food Eng. 2017, 192, 45–52. [Google Scholar] [CrossRef]
- Osborn, B.G.; Fearn, T.; Hindle, P.H. Theory of near infrared spectroscopy. In Practical NIR Spectroscopy with Applications in Food and Beverage Analysis; Longman Singapore Publishiers (Pte) Ltd.: London, UK, 1993; pp. 13–35. [Google Scholar]
- Barbin, D.F.; Badaró, A.T.; Honorato, D.C.B.; Ida, E.Y.; Shimokomaki, M. Identification of Turkey meat and processed products using near infrared spectroscopy. Food Control 2020, 107, 106816. [Google Scholar] [CrossRef]
- Nolasco-Perez, I.M.; Rocco, L.A.C.M.; Cruz-Tirado, J.P.; Pollonio, M.A.R.; Barbon, S.; Barbon, A.P.A.C.; Barbin, D.F. Comparison of rapid techniques for classification of ground meat. Biosyst. Eng. 2019, 183, 151–159. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Alés-Álvarez, F.J.; Torres-García, M.; Feng, C.-H.H.; Álvarez-Mateos, P. Production of oxygenated fuel additives from residual glycerine using biocatalysts obtained from heavy-metal-contaminated Jatropha curcas L. roots. Energies 2019, 12, 740. [Google Scholar] [CrossRef] [Green Version]




| Sample (mg/kg) | Fe | Cr | Cu | Mn | Ni | Pb | Zn | As | Au | Sb |
|---|---|---|---|---|---|---|---|---|---|---|
| Stem | 35.17 | 2.99 | 2.99 | 9.95 | 0.83 | ≤0.4 | 10.29 | ≤0.8 | ≤0.2 | ≤0.2 |
| RSD | 0.57 | 1.24 | 5.76 | 0.22 | 1.37 | - | 0.50 | - | - | - |
| Root | 261.87 | 12.20 | 4.39 | 13.66 | 5.86 | ≤0.2 | 9.27 | ≤0.8 | ≤0.2 | ≤0.2 |
| RSD | 0.59 | 0.72 | 8.10 | 0.41 | 0.61 | - | 0.42 | - | - | - |
| STRATEGY 1: C1—0% Copper, C2—1% Copper, C3—0.5% Copper, and C4—5% Copper | ||||||||||
| Pre-Treatment | Wavelengths | Sensitivity (Validation) | Specificity (Validation) | Accuracy—Calibration Model (%) | ||||||
| C1 | C2 | C3 | C4 | C1 | C2 | C3 | C4 | |||
| Smoothing | 1271, 1376, 1737, 1899, 2013, 2089 | 0.38 | 0.67 | 0.14 | 1.00 | 0.88 | 0.71 | 0.76 | 1.00 | 69.64 |
| Smoothing + SNV | 1147, 1376, 1737, 1851, 1928, 2013, 2118, 2223 | 0.38 | 0.67 | 0.43 | 0.83 | 0.69 | 0.86 | 0.82 | 1.00 | 76.79 |
| Smoothing + first Derivative | 1042, 1166, 1309, 1366, 1480, 1709, 1747, 1813, 1918, 1975, 2061, 2185 | 0.50 | 0.67 | 0.14 | 1.00 | 0.94 | 0.67 | 0.82 | 1.00 | 85.71 |
| Smoothing + second Derivative | 1138, 1271, 1376, 1556, 1709, 1775, 1871, 1947, 2051, 2137, 2232 | 0.38 | 0.33 | 0.14 | 1.00 | 0.69 | 0.86 | 0.71 | 1.00 | 75.00 |
| Smoothing + MSC | 1147, 1376, 1699, 1861, 2013, 2223 | 0.38 | 0.67 | 0.43 | 0.83 | 0.81 | 0.76 | 0.82 | 1.00 | 69.64 |
| Smoothing + MSC + second Derivative | 1081, 1271, 1376, 1556, 1633, 1756, 1851, 1947, 2032, 2108, 2213 | 0.25 | 0.33 | 0.43 | 0.67 | 0.75 | 0.67 | 0.82 | 1.00 | 71.43 |
| Smoothing + SNV + first Derivative | 1042, 1166, 1309, 1480, 1671, 1747, 1899, 2061, 2166 | 0.50 | 0.67 | 0.00 | 0.83 | 0.88 | 0.62 | 0.82 | 1.00 | 85.71 |
| STRATEGY 2: C1—“No Copper” (0%), C2—“Low Content” (0.5-1%) and C3—“High Content” (5%) | ||||||||||
| Pre-Treatment | Wavelengths | Sensitivity (Validation) | Specificity (Validation) | Accuracy—Calibration Model (%) | ||||||
| C1 | C2 | C3 | C1 | C2 | C3 | |||||
| Smoothing | 1271, 1376, 1737, 1899, 2013, 2089 | 0.63 | 0.70 | 1.00 | 0.81 | 0.79 | 1.00 | 78.57 | ||
| Smoothing + SNV | 1147, 1376, 1737, 1851, 1928, 2013, 2118, 2223 | 0.63 | 0.40 | 0.83 | 0.56 | 0.79 | 1.00 | 76.79 | ||
| Smoothing + first Derivative | 1042, 1166, 1309, 1366, 1480, 1709, 1747, 1813, 1918, 1975, 2061, 2185 | 0.63 | 0.70 | 1.00 | 0.81 | 0.79 | 1.00 | 85.71 | ||
| Smoothing + second Derivative | 1138, 1271, 1376, 1556, 1709, 1775, 1871, 1947, 2051, 2137, 2232 | 0.75 | 0.40 | 1.00 | 0.63 | 0.86 | 1.00 | 83.93 | ||
| Smoothing + MSC | 1147, 1376, 1699, 1861, 2013, 2223 | 0.50 | 0.50 | 0.83 | 0.63 | 0.71 | 1.00 | 76.79 | ||
| Smoothing + MSC + second Derivative | 1081, 1271, 1376, 1556, 1633, 1756, 1851, 1947, 2032, 2108, 2213 | 0.50 | 0.50 | 0.67 | 0.63 | 0.64 | 1.00 | 82.14 | ||
| Smoothing + SNV + first Derivative | 1042, 1166, 1309, 1480, 1671, 1747, 1899, 2061, 2166 | 0.75 | 0.70 | 1.00 | 0.81 | 0.86 | 1.00 | 83.93 | ||
| STRATEGY 3: C1—“Low Content” (0–0.5%), C2—“Intermediate Content” (1%) and C3—“High Content” (5%) | ||||||||||
| Pre-Treatment | Wavelengths | Sensitivity (Validation) | Specificity (Validation) | Accuracy—Calibration Model (%) | ||||||
| C1 | C2 | C3 | C1 | C2 | C3 | |||||
| Smoothing | 1271, 1376, 1737, 1899, 2013, 2089 | 0.33 | 0.33 | 1.00 | 0.78 | 0.52 | 1.00 | 69.64 | ||
| Smoothing + SNV | 1147, 1376, 1737, 1851, 1928, 2013, 2118, 2223 | 0.67 | 0.67 | 0.83 | 0.78 | 0.76 | 1.00 | 78.57 | ||
| Smoothing + first Derivative | 1042, 1166, 1309, 1366, 1480, 1709, 1747, 1813, 1918, 1975, 2061, 2185 | 0.40 | 1.00 | 1.00 | 1.00 | 0.57 | 1.00 | 82.14 | ||
| Smoothing + second Derivative | 1138, 1271, 1376, 1556, 1709, 1775, 1871, 1947, 2051, 2137, 2232 | 0.67 | 0.67 | 1.00 | 0.89 | 0.76 | 1.00 | 78.57 | ||
| Smoothing + MSC | 1147, 1376, 1699, 1861, 2013, 2223 | 0.40 | 0.67 | 0.83 | 0.78 | 0.57 | 1.00 | 75.00 | ||
| Smoothing + MSC + second Derivative | 1081, 1271, 1376, 1556, 1633, 1756, 1851, 1947, 2032, 2108, 2213 | 0.53 | 0.33 | 0.67 | 0.78 | 0.57 | 1.00 | 80.36 | ||
| Smoothing + SNV + first Derivative | 1042, 1166, 1309, 1480, 1671, 1747, 1899, 2061, 2166 | 0.47 | 0.67 | 1.00 | 0.89 | 0.62 | 1.00 | 85.71 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Martín, J.F.; Badaró, A.T.; Barbin, D.F.; Álvarez-Mateos, P. Identification of Copper in Stems and Roots of Jatropha curcas L. by Hyperspectral Imaging. Processes 2020, 8, 823. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070823
García-Martín JF, Badaró AT, Barbin DF, Álvarez-Mateos P. Identification of Copper in Stems and Roots of Jatropha curcas L. by Hyperspectral Imaging. Processes. 2020; 8(7):823. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070823
Chicago/Turabian StyleGarcía-Martín, Juan Francisco, Amanda Teixeira Badaró, Douglas Fernandes Barbin, and Paloma Álvarez-Mateos. 2020. "Identification of Copper in Stems and Roots of Jatropha curcas L. by Hyperspectral Imaging" Processes 8, no. 7: 823. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8070823