CO2 Absorption Mechanism by Diamino Protic Ionic Liquids (DPILs) Containing Azolide Anions
Abstract
:1. Introduction
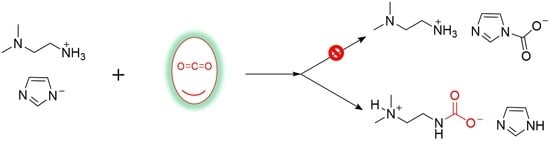
2. Results and Discussion
3. Materials and Methods
3.1. Material and Characterizations
3.2. Synthesis of Ionic Liquids
3.3. CO2 Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef] [Green Version]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Mota-Martinez, M.T.; Hallett, J.P.; Mac Dowell, N. Solvent selection and design for CO2 capture—How we might have been missing the point. Sustain. Energy FUELS 2017, 1, 2078–2090. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Wang, Z.; Bao, Z.; Zhang, Z.; Yang, Y.; Ren, Q.; Xing, H.; Dai, S. New Insights into CO2 Absorption Mechanisms with Amino-Acid Ionic Liquids. Chemsuschem 2016, 9, 806–812. [Google Scholar] [CrossRef]
- Chen, F.-F.; Huang, K.; Zhou, Y.; Tian, Z.-Q.; Zhu, X.; Tao, D.-J.; Jiang, D.-E.; Dai, S. Multi-Molar Absorption of CO2 by the Activation of Carboxylate Groups in Amino Acid Ionic Liquids. Angew. Chem. Int. Ed. 2016, 55, 7166–7170. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Zanatta, M.; Simon, N.M.; dos Santos, F.P.; Corvo, M.C.; Cabrita, E.J.; Dupont, J. Correspondence on “Preorganization and Cooperation for Highly Efficient and Reversible Capture of Low-Concentration CO2 by Ionic Liquids”. Angew. Chem. Int. Ed. 2019, 58, 382–385. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Filippov, A.; Shah, F.U. High CO2 absorption capacity by chemisorption at cations and anions in choline-based ionic liquids. Phys. Chem. Chem. Phys. 2017, 19, 31216–31226. [Google Scholar] [CrossRef] [Green Version]
- Gurkan, B.; Goodrich, B.F.; Mindrup, E.M.; Ficke, L.E.; Massel, M.; Seo, S.; Senftle, T.P.; Wu, H.; Glaser, M.F.; Shah, J.K.; et al. Molecular Design of High Capacity, Low Viscosity, Chemically Tunable Ionic Liquids for CO2 Capture. J. Phys. Chem. Lett. 2010, 1, 3494–3499. [Google Scholar] [CrossRef]
- Wang, C.; Luo, X.; Luo, H.; Jiang, D.-E.; Li, H.; Dai, S. Tuning the Basicity of Ionic Liquids for Equimolar CO2 Capture. Angew. Chem. Int. Ed. 2011, 50, 4918–4922. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Guo, Y.; Ding, F.; Zhao, H.; Cui, G.; Li, H.; Wang, C. Significant Improvements in CO2 Capture by Pyridine-Containing Anion-Functionalized Ionic Liquids through Multiple-Site Cooperative Interactions. Angew. Chem. Int. Ed. 2014, 53, 7053–7057. [Google Scholar] [CrossRef]
- Vijayraghavan, R.; Pas, S.J.; Izgorodina, E.I.; MacFarlane, D.R. Diamino protic ionic liquids for CO2 capture. Phys. Chem. Chem. Phys. 2013, 15, 19994–19999. [Google Scholar] [CrossRef]
- Simons, T.J.; Verheyen, T.; Izgorodina, E.I.; Vijayaraghavan, R.; Young, S.; Pearson, A.K.; Pas, S.J.; MacFarlane, D.R. Mechanisms of low temperature capture and regeneration of CO2 using diamino protic ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 1140–1149. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Tu, Z.; Wu, Y.; Hu, X. Low-viscous diamino protic ionic liquids with fluorine-substituted phenolic anions for improving CO2 reversible capture. J. Mol. Liq. 2018, 268, 617–624. [Google Scholar] [CrossRef]
- Oncsik, T.; Vijayaraghavan, R.; MacFarlane, D.R. High CO2 absorption by diamino protic ionic liquids using azolide anions. Chem. Commun. 2018, 54, 2106–2109. [Google Scholar] [CrossRef]
- Fan, G.-j.; Wee, A.G.H.; Idem, R.; Tontiwachwuthikul, P. NMR Studies of Amine Species in MEA−CO2−H2O System: Modification of the Model of Vapor−Liquid Equilibrium (VLE). Ind. Eng. Chem. Res. 2009, 48, 2717–2720. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Reversible carbon dioxide capture by aqueous and non-aqueous amine-based absorbents: A comparative analysis carried out by 13C NMR spectroscopy. Appl. Energy 2018, 220, 208–219. [Google Scholar] [CrossRef]
- Lei, X.; Xu, Y.; Zhu, L.; Wang, X. Highly efficient and reversible CO2 capture through 1,1,3,3-tetramethylguanidinium imidazole ionic liquid. Rsc. Adv. 2014, 4, 7052–7057. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Z.; Ji, P.; Cheng, J.-P. CO2 Absorption by DBU-Based Protic Ionic Liquids: Basicity of Anion Dictates the Absorption Capacity and Mechanism. Front. Chem. 2019, 6, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukesh, C.; Khokarale, S.G.; Virtanen, P.; Mikkola, J.-P. Rapid desorption of CO2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential. Sustain. Energy Fuels 2019, 3, 2125–2134. [Google Scholar] [CrossRef]
- Yu, B.; Li, L.; Yu, H.; Maeder, M.; Puxty, G.; Yang, Q.; Feron, P.; Conway, W.; Chen, Z. Insights into the Chemical Mechanism for CO2(aq) and H+ in Aqueous Diamine Solutions—An Experimental Stopped-Flow Kinetic and 1H/13C NMR Study of Aqueous Solutions of N,N-Dimethylethylenediamine for Postcombustion CO2 Capture. Environ. Sci. Technol. 2018, 52, 916–926. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wu, C.; Yang, D. CO2 Absorption Mechanism by Diamino Protic Ionic Liquids (DPILs) Containing Azolide Anions. Processes 2021, 9, 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9061023
Wang X, Wu C, Yang D. CO2 Absorption Mechanism by Diamino Protic Ionic Liquids (DPILs) Containing Azolide Anions. Processes. 2021; 9(6):1023. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9061023
Chicago/Turabian StyleWang, Xiao, Congyi Wu, and Dezhong Yang. 2021. "CO2 Absorption Mechanism by Diamino Protic Ionic Liquids (DPILs) Containing Azolide Anions" Processes 9, no. 6: 1023. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9061023