Using Remote Sensing to Identify Drivers behind Spatial Patterns in the Bio-physical Properties of a Saltmarsh Pioneer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area
2.2. In situ Measurements
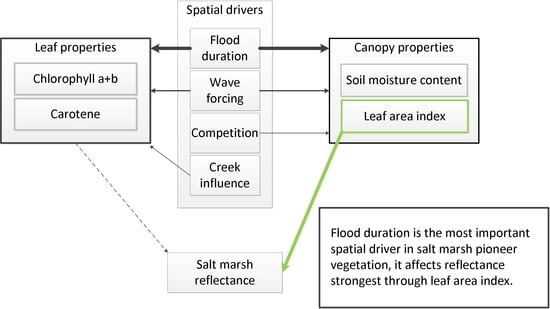
2.3. Spatial Drivers
2.4. Model
2.5. Model Inversion
2.6. Sensitivity Modeled Vegetation Characteristics
2.7. Model Validation
2.8. Application to Spaceborne Data
3. Results
3.1. Effects Spatial Drivers on in situ Vegetation Characteristics
3.2. Effects of Vegetation Characteristics on Reflectance, Modelled Sensitivity
3.3. Model Validation
3.4. Large Scale Effect of Spatial Drivers
4. Discussion
4.1. Applicability to Other Vegetation Zones
4.2. ProSail
4.3. Effect of Spatial Drivers on Leaf and Canopy Level
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Legendre, P.; Fortin, M.J. Spatial pattern and ecological analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef] [Green Version]
- Bertness, M.D.; Ellison, A.M. Determinants of pattern in a New England salt marsh plant community. Ecol. Monogr. 1987, 129–147. [Google Scholar] [CrossRef]
- Kéfi, S.; Dakos, V.; Scheffer, M.; Van Nes, E.H.; Rietkerk, M. Early warning signals also precede non-catastrophic transitions. Oikos 2013, 122, 641–648. [Google Scholar] [CrossRef]
- van Belzen, J.; van de Koppel, J.; Kirwan, M.L.; van der Wal, D.; Herman, P.M.J.; Dakos, V.; Kéfi, S.; Scheffer, M.; Guntenspergen, G.R.; Bouma, T.J. Vegetation recovery in tidal marshes reveals critical slowing down under increased inundation. Nat. Commun. 2017, 8, ncomms15811. [Google Scholar] [CrossRef] [PubMed]
- Marani, M.; Da Lio, C.; D’Alpaos, A. Vegetation engineers marsh morphology through multiple competing stable states. Proc. Natl. Acad. Sci. 2013, 110, 3259–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, A.; Ganguly, S.; Hashimoto, H.; Devadiga, S.; Vermote, E.; Knyazikhin, Y.; Nemani, R.R.; Myneni, R.B. Amazon forests did not green-up during the 2005 drought. Geophys. Res. Lett. 2010, 37. [Google Scholar] [CrossRef] [Green Version]
- Murad, H.; Islam, A. Drought assessment using remote sensing and GIS in north-west region of Bangladesh. In Proceedings of the Proceedings of the 3rd International Conference on Water & Flood Management, Dhaka, Bangladesh, 8–10 January 2011; pp. 797–804. [Google Scholar]
- Caccamo, G.; Chisholm, L.A.; Bradstock, R.A.; Puotinen, M.L. Assessing the sensitivity of MODIS to monitor drought in high biomass ecosystems. Remote Sens. Environ. 2011, 115, 2626–2639. [Google Scholar] [CrossRef]
- Aldakheel, Y.Y. Assessing NDVI spatial pattern as related to irrigation and soil salinity management in Al-Hassa Oasis, Saudi Arabia. J. indian Soc. Remote Sens. 2011, 39, 171–180. [Google Scholar] [CrossRef]
- Lobell, D.B.; Lesch, S.M.; Corwin, D.L.; Ulmer, M.G.; Anderson, K.A.; Potts, D.J.; Doolittle, J.A.; Matos, M.R.; Baltes, M.J. Regional-scale assessment of soil salinity in the Red River Valley using multi-year MODIS EVI and NDVI. J. Environ. Qual. 2010, 39, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Belluco, E.; Camuffo, M.; Ferrari, S.; Modenese, L.; Silvestri, S.; Marani, A.; Marani, M. Mapping salt-marsh vegetation by multispectral and hyperspectral remote sensing. Remote Sens. Environ. 2006, 105, 54–67. [Google Scholar] [CrossRef]
- Berger, K.; Atzberger, C.; Danner, M.; D’Urso, G.; Mauser, W.; Vuolo, F.; Hank, T. Evaluation of the PROSAIL model capabilities for future hyperspectral model environments: a review study. Remote Sens. 2018, 10, 85. [Google Scholar] [CrossRef]
- Tripathi, R.; Sahoo, R.N.; Sehgal, V.K.; Tomar, R.K.; Chakraborty, D.; Nagarajan, S. Inversion of PROSAIL model for retrieval of plant biophysical parameters. J. Indian Soc. Remote Sens. 2012, 40, 19–28. [Google Scholar] [CrossRef]
- Jacquemoud, S. Inversion of the PROSPECT+ SAIL canopy reflectance model from AVIRIS equivalent spectra: theoretical study. Remote Sens. Environ. 1993, 44, 281–292. [Google Scholar] [CrossRef]
- Bicheron, P.; Leroy, M. A method of biophysical parameter retrieval at global scale by inversion of a vegetation reflectance model. Remote Sens. Environ. 1999, 67, 251–266. [Google Scholar] [CrossRef]
- Boesch, D.F.; Turner, R.E. Dependence of fishery species on salt marshes: the role of food and refuge. Estuaries 1984, 7, 460–468. [Google Scholar] [CrossRef]
- Deegan, L.A.; Hughes, J.E.; Rountree, R.A. Salt marsh ecosystem support of marine transient species. In Concepts and controversies in tidal marsh ecology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 333–365. ISBN 0792360192. [Google Scholar]
- Chmura, G.L. What do we need to assess the sustainability of the tidal salt marsh carbon sink? Ocean Coast. Manag. 2011. [Google Scholar] [CrossRef]
- Henderson, F.M.; Lewis, A.J. Radar detection of wetland ecosystems: a review. Int. J. Remote Sens. 2008, 29, 5809–5835. [Google Scholar] [CrossRef] [Green Version]
- Möller, I. Quantifying saltmarsh vegetation and its effect on wave height dissipation: Results from a UK East coast saltmarsh. Estuar. Coast. Shelf Sci. 2006, 69, 337–351. [Google Scholar] [CrossRef]
- Barbier, E.B.; Koch, E.W.; Silliman, B.R.; Hacker, S.D.; Wolanski, E.; Primavera, J.; Granek, E.F.; Polasky, S.; Aswani, S.; Cramer, L.A. Coastal ecosystem-based management with nonlinear ecological functions and values. Science 2008, 319, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Möller, I.; Kudella, M.; Rupprecht, F.; Spencer, T.; Paul, M.; Van Wesenbeeck, B.K.; Wolters, G.; Jensen, K.; Bouma, T.J.; Miranda-Lange, M. Wave attenuation over coastal salt marshes under storm surge conditions. Nat. Geosci. 2014, 7, 727–731. [Google Scholar] [CrossRef]
- Rönnbäck, P. The ecological basis for economic value of seafood production supported by mangrove ecosystems. Ecol. Econ. 1999, 29, 235–252. [Google Scholar] [CrossRef]
- Jerath, M.; Bhat, M.; Rivera-Monroy, V.H.; Castañeda-Moya, E.; Simard, M.; Twilley, R.R. The role of economic, policy, and ecological factors in estimating the value of carbon stocks in Everglades mangrove forests, South Florida, USA. Environ. Sci. Policy 2016, 66, 160–169. [Google Scholar] [CrossRef]
- Rizal, A.; Sahidin, A.; Herawati, H. Economic Value Estimation of Mangrove Ecosystems in Indonesia. Biodivers. Int. J. 2018, 2, 51. [Google Scholar] [CrossRef]
- Zedler, J.B.; Callaway, J.C.; Desmond, J.S.; Vivian-Smith, G.; Williams, G.D.; Sullivan, G.; Brewster, A.E.; Bradshaw, B.K. Californian salt-marsh vegetation: an improved model of spatial pattern. Ecosystems 1999, 2, 19–35. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Foin, T.C.; Ustin, S.L. A simple empirical model of salt marsh plant spatial distributions with respect to a tidal channel network. Ecol. Modell. 2001, 139, 293–307. [Google Scholar] [CrossRef]
- Silvestri, S.; Marani, M. Salt-Marsh Vegetation and Morphology: Basic Physiology, Modelling and Remote Sensing Observations. Ecogeomorphology Tidal Marshes 2004, 5–25. [Google Scholar]
- Pettengill, T.M.; Crotty, S.M.; Angelini, C.; Bertness, M.D. A natural history model of New England salt marsh die-off. Oecologia 2018, 186, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Moffett, K.B.; Robinson, D.A.; Gorelick, S.M. Relationship of salt marsh vegetation zonation to spatial patterns in soil moisture, salinity, and topography. Ecosystems 2010, 13, 1287–1302. [Google Scholar] [CrossRef]
- Silvestri, S.; Defina, A.; Marani, M. Tidal regime, salinity and salt marsh plant zonation. Estuar. Coast. Shelf Sci. 2005, 62, 119–130. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. Salt marsh plant zonation: the relative importance of competition and physical factors. Ecology 1992, 73, 681–690. [Google Scholar] [CrossRef]
- Neumeier, U.; Ciavola, P. Flow resistance and associated sedimentary processes in a Spartina maritima salt-marsh. J. Coast. Res. 2004, 435–447. [Google Scholar] [CrossRef]
- Bouma, T.J.; De Vries, M.B.; Low, E.; Peralta, G.; Tánczos, I.C.; van de Koppel, J.; Herman, P.M.J. Trade-offs related to ecosystem engineering: A case study on stiffness of emerging macrophytes. Ecology 2005, 86, 2187–2199. [Google Scholar] [CrossRef]
- Bouma, T.J.; De Vries, M.B.; Herman, P.M.J. Comparing ecosystem engineering efficiency of two plant species with contrasting growth strategies. Ecology 2010, 91, 2696–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaghan, D.P.; Bouma, T.J.; Klaassen, P.; Van der Wal, D.; Stive, M.J.F.F.; Herman, P.M.J.J. Hydrodynamic forcing on salt-marsh development: Distinguishing the relative importance of waves and tidal flows. Estuar. Coast. Shelf Sci. 2010, 89, 73–88. [Google Scholar] [CrossRef]
- Fagherazzi, S.; Carniello, L.; D’Alpaos, L.; Defina, A. Critical bifurcation of shallow microtidal landforms in tidal flats and salt marshes. Proc. Natl. Acad. Sci. 2006, 103, 8337–8341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertness, M.D.; Hacker, S.D. Physical stress and positive associations among marsh plants. Am. Nat. 1994, 363–372. [Google Scholar] [CrossRef]
- Emery, N.C.; Ewanchuk, P.J.; Bertness, M.D. Competition and salt-marsh plant zonation: stress tolerators may be dominant competitors. Ecology 2001, 82, 2471–2485. [Google Scholar] [CrossRef]
- Xin, P.; Li, L.; Barry, D.A. Tidal influence on soil conditions in an intertidal creek-marsh system. Water Resour. Res. 2013. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Liu, Q.; Lu, Q.; Gao, Z.; Wang, J. Spatial and Seasonal Variations of Soil Carbon and Nitrogen Content and Stock in a Tidal Salt Marsh with Tamarix chinensis, China. Wetlands 2016, 36, 145–152. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Van der Wal, D.; Wielemaker-Van den Dool, A.; Herman, P.M.J. Spatial patterns, rates and mechanisms of saltmarsh cycles (Westerschelde, The Netherlands). Estuar. Coast. Shelf Sci. 2008, 76, 357–368. [Google Scholar] [CrossRef]
- Van Der Wal, D.; Herman, P.M.J. Regression-based synergy of optical, shortwave infrared and microwave remote sensing for monitoring the grain-size of intertidal sediments. Remote Sens. Environ. 2007, 111, 89–106. [Google Scholar] [CrossRef]
- Van Damme, S.; Struyf, E.; Maris, T.; Ysebaert, T.; Dehairs, F.; Tackx, M.; Heip, C.; Meire, P. Spatial and temporal patterns of water quality along the estuarine salinity gradient of the Scheldt estuary (Belgium and The Netherlands): results of an integrated monitoring approach. Hydrobiologia 2005, 540, 29–45. [Google Scholar] [CrossRef]
- Wang, H.; Wal, D.; Li, X.; Belzen, J.; Herman, P.M.J.; Hu, Z.; Ge, Z.; Zhang, L.; Bouma, T.J. Zooming in and out: scale-dependence of extrinsic and intrinsic factors affecting salt marsh erosion. J. Geophys. Res. Earth Surf. 2017. [Google Scholar] [CrossRef]
- Kromkamp, J.C.; Morris, E.P.; Forster, R.M.; Honeywill, C.; Hagerthey, S.; Paterson, D.M. Relationship of intertidal surface sediment chlorophyll concentration to hyperspectral reflectance and chlorophyll fluorescence. Estuaries Coasts 2006, 29, 183–196. [Google Scholar] [CrossRef]
- Van der Wal, D.; Herman, P.M.J.; Forster, R.M.; Ysebaert, T.; Rossi, F.; Knaeps, E.; Plancke, Y.M.G.; Ides, S.J. Distribution and dynamics of intertidal macrobenthos predicted from remote sensing: response to microphytobenthos and environment. Mar. Ecol. Prog. Ser. 2008, 367, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Paree, E. Toelichting op de zoute ecotopenkaart Westerschelde 2016 - Biologische monitoring zoute rijkswateren; Rijkswaterstaat Ministerie Infrastructuur en Milieu: The Hague, The Netherlands, 2017; 25p. [Google Scholar]
- Tolman, M.E.; Pranger, D.P. Toelichting bij de Vegetatiekartering Westerschelde 2010. Rijkswaterstaat Minist. van verkeer en Waterstaat Delft 2012, 1–114. Available online: http://publicaties.minienm.nl/documenten/toelichting-bij-de-vegetatiekartering-westerschelde-2010-op-basi (accessed on 19 February 2019).
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: the SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+ SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Lehnert L., W.; Meyer, H.; Bendix, J. HSDAR: Manage, analyse and simulate hyperspectral data in R. R package version 0.5.0. (2016). Available online: https://rdrr.io/cran/hsdar/ (accessed on 1 March 2019).
- Bowyer, P.; Danson, F.M. Sensitivity of spectral reflectance to variation in live fuel moisture content at leaf and canopy level. Remote Sens. Environ. 2004, 92, 297–308. [Google Scholar] [CrossRef]
- Mobasheri, M.R.; Fatemi, S.B. Leaf Equivalent Water Thickness assessment using reflectance at optimum wavelengths. Theor. Exp. Plant Physiol. 2013, 25, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Beleites, C.; Sergo, V. HyperSpec: a package to handle hyperspectral data sets in R’, R package version 0.98-20150304. Available online: http://hyperspec.r-forge.r-project.org (accessed on 1 August 2017).
- Jensen, J.R.; Coombs, C.; Porter, D.; Jones, B.; Schill, S.; White, D. Extraction of smooth cordgrass (Spartina alterniflora) biomass and leaf area index parameters from high resolution imagery. Geocarto Int. 1998, 13, 25–34. [Google Scholar] [CrossRef]
- Van der Meijden, R. Heukels’ Flora van Nederland 22e druk; Wolter-Noordhof: Groningen, The Netherlands, 1996. [Google Scholar]
- Morris, J.T. Modelling light distribution within the canopy of the marsh grass Spartina alterniflora as a function of canopy biomass and solar angle. Agric. For. Meteorol. 1989, 46, 349–361. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The spectral image processing system (SIPS)—interactive visualization and analysis of imaging spectrometer data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Kotchenova, S.Y.; Vermote, E.F. Validation of a vector version of the 6S radiative transfer code for atmospheric correction of satellite data Part II Homogeneous Lambertian and anisotropic surfaces. Appl. Opt. 2007, 46, 4455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feagin, R.A.; Martinez, M.L.; Mendoza-Gonzalez, G.; Costanza, R. Salt marsh zonal migration and ecosystem service change in response to global sea level rise: a case study from an urban region. Ecol. Soc. 2010, 15. [Google Scholar] [CrossRef]
- Zheng, G.; Moskal, L.M. Retrieving leaf area index (LAI) using remote sensing: theories, methods and sensors. Sensors 2009, 9, 2719–2745. [Google Scholar] [CrossRef] [PubMed]
- Verger, A.; Baret, F.; Camacho, F. Optimal modalities for radiative transfer-neural network estimation of canopy biophysical characteristics: Evaluation over an agricultural area with CHRIS/PROBA observations. Remote Sens. Environ. 2011, 115, 415–426. [Google Scholar] [CrossRef]
- Tyc, G.; Tulip, J.; Schulten, D.; Krischke, M.; Oxfort, M. The RapidEye mission design. Acta Astronaut. 2005, 56, 213–219. [Google Scholar] [CrossRef]
- Sozzi, M.; Marinello, F.; Pezzuolo, A.; Sartori, L. Benchmark of Satellites Image Services for Precision Agricultural use. In Proceedings of the AgEng Conference, Wageningen, The Netherlands, 8–11 July 2018. [Google Scholar]
- Botha, E.J.; Leblon, B.; Zebarth, B.; Watmough, J. Non-destructive estimation of potato leaf chlorophyll from canopy hyperspectral reflectance using the inverted PROSAIL model. Int. J. Appl. Earth Obs. Geoinf. 2007, 9, 360–374. [Google Scholar] [CrossRef]
- Tripathi, R.; Sahoo, R.N.; Sehgal, V.K.; Gupta, V.K.; Bhattacharya, B.B.K.; Gupta, K.; Bhattacharya, B.B.K. Remote Sensing Derived Composite Vegetation Health Index Through Inversion of Prosail for Monitoring of Wheat Growth in Trans Gangetic Plains of India. ISPRS Arch. XXXVIII-8/W3 Work. Proc. Impact Clim. Chang. Agric. 2009, 319–325. [Google Scholar]
- Duan, S.-B.; Li, Z.-L.; Wu, H.; Tang, B.-H.; Ma, L.; Zhao, E.; Li, C. Inversion of the PROSAIL model to estimate leaf area index of maize, potato, and sunflower fields from unmanned aerial vehicle hyperspectral data. Int. J. Appl. Earth Obs. Geoinf. 2014, 26, 12–20. [Google Scholar] [CrossRef]
- Si, Y.; Schlerf, M.; Zurita-Milla, R.; Skidmore, A.; Wang, T. Mapping spatio-temporal variation of grassland quantity and quality using MERIS data and the PROSAIL model. Remote Sens. Environ. 2012, 121, 415–425. [Google Scholar] [CrossRef]
- Li, Z.; Jin, X.; Wang, J.; Yang, G.; Nie, C.; Xu, X.; Feng, H. Estimating winter wheat (Triticum aestivum) LAI and leaf chlorophyll content from canopy reflectance data by integrating agronomic prior knowledge with the PROSAIL model. Int. J. Remote Sens. 2015, 36, 2634–2653. [Google Scholar] [CrossRef]
- Kooistra, L.; Clevers, J.G.P.W. Estimating potato leaf chlorophyll content using ratio vegetation indices. Remote Sens. Lett. 2016, 7, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Kearney, M.S.; Stutzer, D.; Turpie, K.; Stevenson, J.C. The effects of tidal inundation on the reflectance characteristics of coastal marsh vegetation. J. Coast. Res. 2009, 1177–1186. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo, J.M.; Fernández-Baco, L.; Castellanos, E.M.; Luque, C.J.; Figueroa, M.E.; Davy, A.J. Lower limits of Spartina densiflora and S. maritima in a Mediterranean salt marsh determined by different ecophysiological tolerances. J. Ecol. 2000, 88, 801–812. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation stress: an introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]





| Model | Parameter Name | Model Abbreviation | Mean Value | In Situ Range | Source |
|---|---|---|---|---|---|
| Prospect | Structure parameter | N | Fixed (1.5) | Fixed | Fitted + [42] |
| Chlorophyll a+b content | Cab (µg/cm2) | 56.4 | 42.4–76.5 | Chl samples | |
| Carotenoid content | Car (µg/cm2) | 3.421 | 2.398–4.579 | Chl samples | |
| Brown pigment content | Cbrown | N.A. | N.A. | - | |
| Equivalent water thickness | Cw (cm) | Fixed (0.0198) | Fixed | leaf samples | |
| Dry matter content | Cm (g/cm2) | Fixed (0.0092) | Fixed | Leaf samples | |
| Sail | Dry/Wet soil fraction (=1- soil moisture content) | pSoil | 0.5340 | 0.4496–0.6214 | Soil samples |
| Leaf area index | LAI | 0.706 | 0.003-1.215 | [57] + samples | |
| Type of leaf angle distribution | Lidf | Fixed(1,0) | Fixed | [59] | |
| Hotspot parameter | hspot | N.A. | N.A. | - | |
| Solar zenith angle | Tts (°) | N.A. | N.A. | From timestamp | |
| Observer zenith angle | Tto (°) | N.A. | N.A. | Always 0 | |
| Relative azimuth angle | Psi (°) | N.A. | N.A. | From timestamp |
| Parameter | Setting |
|---|---|
| Month | 06, from satellite image |
| Day | 05, from satellite image |
| Solar zentih angle (deg) | 28.91, from satellite image |
| Solar azimuth angle (deg) | 171.91, rom satellite image |
| Sensor zenith angle (deg) | 12.79, from satellite image |
| Sensor azimuth angle (deg) | 281.32, from satellite image |
| Atmospheric profile | Mid latitude summer/winter, here summer |
| Aerosol profile | Maritime |
| Target altitude | Sea level |
| Sensor altitude | Satellite level |
| Spectral conditions | RapidEye gain, band 1-5 |
| Ground reflectance | Homogeneous surface |
| Directional effects | No directional effects |
| Input ground reflectance | Mean spectral value |
| Atmospheric correction mode | Atmospheric correction with Lambertian assumption |
| Atmospheric correction target | 0, Reflectance |
| Parameter | Minimum | Maximum | Stepsize |
|---|---|---|---|
| LAI | 0.001 | 3 | 0.01 |
| Chl-ab (µg/cm2) | 1 | 100 | 0.1 |
| pSoil | 0.1 | 1 | 0.001 |
| . | Flood Duration | Wave Forcing | Creek Influence | Competition |
|---|---|---|---|---|
| Flood duration | 1.00 | −0.44 | 0.55 | 0.41 |
| Wave forcing | −0.44 | 1.00 | −0.30 | −0.08 |
| Creek influence | 0.55 | −0.30 | 1.00 | 0.41 |
| Competition | 0.41 | −0.08 | 0.41 | 1.00 |
| Spatial Driver | Absolute Contribution to t-Value | Coefficient |
|---|---|---|
| Flood Duration | 67.743 | −0.01078 |
| Wave Forcing | 9.124 | 0.00033 |
| Creek Influence | 2.321 | 0.00007 |
| Competition | 0.900 | 0.00007 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oteman, B.; Morris, E.P.; Peralta, G.; Bouma, T.J.; van der Wal, D. Using Remote Sensing to Identify Drivers behind Spatial Patterns in the Bio-physical Properties of a Saltmarsh Pioneer. Remote Sens. 2019, 11, 511. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050511
Oteman B, Morris EP, Peralta G, Bouma TJ, van der Wal D. Using Remote Sensing to Identify Drivers behind Spatial Patterns in the Bio-physical Properties of a Saltmarsh Pioneer. Remote Sensing. 2019; 11(5):511. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050511
Chicago/Turabian StyleOteman, Bas, Edward Peter Morris, Gloria Peralta, Tjeerd Joris Bouma, and Daphne van der Wal. 2019. "Using Remote Sensing to Identify Drivers behind Spatial Patterns in the Bio-physical Properties of a Saltmarsh Pioneer" Remote Sensing 11, no. 5: 511. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11050511