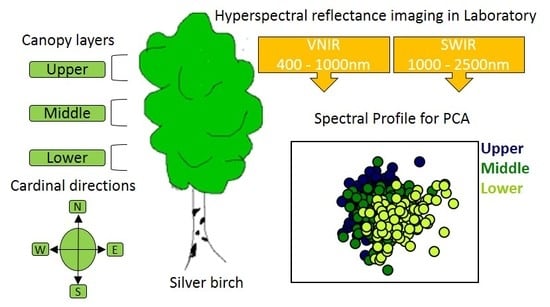
Leaf Canopy Layers Affect Spectral Reflectance in Silver Birch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Sampling
2.2. Leaf Trait Measurements
2.3. Reflectance Spectral Measurements
2.4. Spectral Preprocessing
2.5. Reflectance Indices
2.6. Analysis and Classification
3. Results
3.1. Differences in Reflectance Among Canopy Layers
3.2. Wavelengths Discriminating the Canopy Layers
3.3. Differences in Reflectance Among The Cardinal Directions
3.4. Red Edge, NDVI, and PRI Variation among Canopy Layers and Cardinal Directions
3.5. Leaf Traits among the Canopy Layers and Cardinal Directions
3.6. Relationship between Leaf Traits and Their Reflectance Indices among the Canopy Layers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niinemets, U. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef] [PubMed]
- Shvidenko, A.; Barber, C.V.; Persson, R.; Gonzalez, P.; Hassan, R.; Lakyda, P. Forest and Woodland Systems. In Current State & Trends Assessment of the Millennium Assessment; Island Press: Washington, DC, USA, 2005; Volume 1, pp. 587–621. [Google Scholar]
- Atkinson, M.D. Betula pendula Roth (B. verrucosa Ehrh) and B. pubescens Ehrh. J. Ecol. 1992, 80, 837–870. [Google Scholar] [CrossRef]
- Rijkers, T.; Pons, T.L.; Bongers, F. The effect of tree height and light availability on photosynthetic leaf traits of four neotropical species differing in shade tolerance. Funct. Ecol. 2000, 14, 77–86. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Cescatti, A.; Rodeghiero, M.; Tosens, T. Complex adjustments of photosynthetic potentials and internal diffusion conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ. 2006, 29, 1159–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Violle, C.; Enquist, B.J.; McGill, B.J.; Jiang, L.; Albert, C.H.; Hulshof, C.; Jung, V.; Messier, J. The return of the variance: Intraspecific variability in community ecology. Trends Ecol. Evol. 2012, 27, 244–252. [Google Scholar] [CrossRef]
- Deepak, M.; Lihavainen, J.; Keski-Saari, S.; Kontunen-Soppela, S.; Salojärvi, J.; Tenkanen, A.; Heimonen, K.; Oksanen, E.; Keinänen, M. Genotype-and provenance-related variation in the leaf surface secondary metabolites of silver birch. Can. J. For. Res. 2018, 48, 494–505. [Google Scholar] [CrossRef] [Green Version]
- Possen, B.J.H.M.; Anttonen, M.J.; Oksanen, E.; Rousi, M.; Heinonen, J.; Kostiainen, K.; Kontunen-Soppela, S.; Heiskanen, J.; Vapaavuori, E. Variation in 13 leaf morphological and physiological traits within a silver birch (Betula pendula) stand and their relation to growth. Can. J. For. Res. 2014, 44, 657–665. [Google Scholar] [CrossRef]
- Petruzzellis, F.; Palandrani, C.; Savi, T.; Alberti, R.; Nardini, A.; Bacaro, G. Sampling intraspecific variability in leaf functional traits: Practical suggestions to maximize collected information. Ecol. Evol. 2017, 7, 11236–11245. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C.; Döll, M.; Fietz, H.-J.; Bach, T.; Kozel, U.; Meier, D.; Rahmsdorf, U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981, 2, 115–141. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Marek, M.V.; Kalina, J.; Urban, O. Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. J. Plant Physiol. Biochem. 2007, 45, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Optimal nitrogen distribution within a leaf canopy under direct and diffuse light. Plant Cell Environ. 2014, 37, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Babani, F.; Langsdorf, G. Chlorophyll fluorescence imaging of photosynthetic activity in sun and shade leaves of trees. Photosynth. Res. 2007, 93, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Gara, T.W.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T. Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species. Remote Sens. 2018, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Poorter, L.; Oberbauer, S.F.; Clark, D.B. Leaf optical properties along a vertical gradient in a tropical rainforest canopy in Costa Rica. Am. J. Bot. 1995, 82, 1257–1263. [Google Scholar] [CrossRef]
- Atherton, J.; Olascoaga, B.; Alonso, L.; Porcar-Castell, A. Spatial variation of leaf optical properties in a boreal forest is influenced by species and light environment. Front. Plant Sci. 2017, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Scartazza, A.; Di Baccio, D.; Bertolotto, P.; Gavrichkova, O.; Matteucci, G. Investigating the European beech (Fagus sylvatica L.) leaf characteristics along the vertical canopy profile: Leaf structure, photosynthetic capacity, light energy dissipation and photoprotection mechanisms. Tree Physiol. 2016, 36, 1060–1076. [Google Scholar] [CrossRef] [Green Version]
- Sellin, A.; Rosenvald, K.; Õunapuu-Pikas, E.; Tullus, A.; Ostonen, I.; Lõhmus, K. Elevated air humidity affects hydraulic traits and tree size but not biomass allocation in young silver birches (Betula pendula). Front. Plant Sci. 2015, 6, 860. [Google Scholar] [CrossRef] [Green Version]
- Khavaninzadeh, A.R.; Veroustraete, F.; Van Wittenberghe, S.; Verrelst, J.; Samson, R. Leaf reflectance variation along a vertical crown gradient of two deciduous tree species in a Belgian industrial habitat. Environ. Pollut. 2015, 204, 324–332. [Google Scholar] [CrossRef]
- Buajan, S.; Jinfu, L.; ZhongSheng, H.; XuePing, F.; Muhammad, A. The effect of light on microenvironment and specific leaf area within the gap, subtropical forest. China Pak. J. Bot. 2017, 49, 273–282. [Google Scholar]
- Carswell, F.E.; Meir, P.; Wandelli, E.V.; Bonates, L.C.M.; Kruijt, B.; Barbosa, E.M.; Nobre, A.D.; Grace, J.; Jarvis, P.G. Photosynthetic capacity in a central Amazonian rain forest. Tree Physiol. 1999, 20, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral properties of plants. Appl. Opt. 1965, 4, 11–20. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2010, 189, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Hovi, A.; Raitio, P.; Rautiainen, M. A spectral analysis of 25 boreal tree species. Silva Fennica 2017, 51, 4. [Google Scholar] [CrossRef] [Green Version]
- Cavender-Bares, J.; Meireles, J.; Couture, J.; Kaproth, M.; Kingdon, C.; Singh, A.; Serbin, S.; Center, A.; Zuniga, E.; Pilz, G.; et al. Associations of leaf spectra with genetic and phylogenetic variation in oaks: Prospects for remote detection of biodiversity. Remote Sens. 2016, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Danusevicius, D.; Masaitis, G.; Mozgeris, G. Visible and near infrared hyperspectral imaging reveals significant differences in needle reflectance among Scots pine provenances. Silvae Genet. 2014, 63, 169–180. [Google Scholar] [CrossRef] [Green Version]
- Čepl, J.; Stejskal, J.; Lhotáková, Z.; Holá, D.; Korecký, J.; Lstibůrek, M.; Tomášková, I.; Kočová, M.; Rothová, O.; Palovská, M.; et al. Heritable variation in needle spectral reflectance of Scots pine (Pinus sylvestris L.) peaks in red edge. 2018. Remote Sens. Environ. 2018, 219, 89–98. [Google Scholar] [CrossRef]
- Baránková, B.; Lazár, D.; Nauš, J. Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves. Remote Sens. Environ. 2016, 174, 181–196. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Lukeš, P.; Stenberg, P.; Rautiainen, M.; Mõttus, M.; Vanhatalo, K.M. Optical properties of leaves and needles for boreal tree species in Europe. Remote Sens. Lett. 2013, 4, 667–676. [Google Scholar] [CrossRef]
- Rautiainen, M.; Lukeš, P.; Homolová, L.; Hovi, A.; Pisek, J.; Mõttus, M. Spectral Properties of Coniferous Forests: A Review of In Situ and Laboratory Measurements. Remote Sens. 2018, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Sonobe, R.; Wang, Q. Hyperspectral indices for quantifying leaf chlorophyll concentrations performed differently with different leaf types in deciduous forests. Ecol. Inform. 2017, 37, 1–9. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation-use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Gamon, J.A.; Bond, B. Effects of irradiance and photosynthetic downregulation on the photochemical reflectance index in Douglas-fir and ponderosa pine. Remote Sens. Environ. 2013, 135, 141–149. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Anderson, C.B.; Knapp, D.E. Quantifying forest canopy traits: Imaging spectroscopy versus field survey. Remote Sens. Environ. 2015, 158, 15–27. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M. Leaf Pigment Content. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Oxford, UK, 2017. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Verma, S.B.; Rundquist, D.C.; Arkebauer, T.J.; Keydan, G. Relationship between gross primary production and chlorophyll content in crops: Implications for the synoptic monitoring of vegetation productivity. J. Geophys. Res. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Sampson, P.H.; Zarco-Tejada, P.J.; Mohammed, G.H.; Miller, J.R.; Noland, T.L. Hyperspectral remote sensing of forest condition: Estimating chlorophyll content in tolerant hardwoods. For. Sci. 2003, 49, 381–391. [Google Scholar] [CrossRef]
- Gara, T.W.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Heurich, M. Accurate modelling of canopy traits from seasonal Sentinel-2 imagery based on the vertical distribution of leaf traits. ISPRS J. Photogramm. Remote Sens. 2019, 157, 108–123. [Google Scholar] [CrossRef]
- Weiskittel, A.R.; Temesgen, H.; Wilson, D.; Maguire, D.A. Sources of within-and between-stand variability in specific leaf area of three ecologically distinct conifer species. Ann. For. Sci. 2008, 65, 1–10. [Google Scholar] [CrossRef]
- Schittenhelm, J.; Toder, S.; Fath, S.; Westphal, S.; Wagner, E. Photoinactivation of catalase in needles of Norway spruce. Physiol. Plant. 1994, 90, 600–606. [Google Scholar] [CrossRef]
- Lhotáková, Z.; Albrechtová, J.; Malenovský, Z.; Rock, B.N.; Polák, T.; Cudlín, P. Does the azimuth orientation of Norway spruce (Picea abies/L./Karst.) branches within sunlit crown part influence the heterogeneity of biochemical, structural and spectral characteristics of needles. Environ. Exp. Bot. 2007, 59, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Heimonen, K.; Valtonen, A.; Kontunen-Soppela, S.; Keski-Saari, S.; Rousi, M.; Oksanen, E.; Roininen, H. Colonization of a host tree by herbivorous insects under a changing climate. Oikos 2015, 124, 1013–1022. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ben Ghozlen, N.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on Dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.R.; Hare, E.W.; Wu, J. Quantitative characterisation of the red edge reflectance 1. An inverted-Gaussian model. Int. J. Remote Sens. 1990, 11, 1755–1773. [Google Scholar] [CrossRef]
- Boochs, F.; Kupfer, G.; Dockter, K.; Kuhbauch, W. Shape of the red edge as vitality indicator for plants. Int. J. Remote Sens. 1990, 11, 1741–1753. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F-735/F-700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a+b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Birth, G.; McVey, G. Measuring the color of growing turf with a reflectance spectrophotometer. Agron. J. 1968, 60, 640–643. [Google Scholar] [CrossRef]
- Datt, B. A new reflectance index for remote sensing of chlorophyll content in higher plants: Tests using eucalyptus leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berni, J.A.J.; Suarez, L.; Sepulcre-Canto, G.; Morales, F.; Miller, J.R. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113(6), 1262–1275. [Google Scholar] [CrossRef]
- Gong, Z.; Zhao, Y.; Zhao, W.; Lin, C. Estimation model for plant leaf chlorophyll content based on the spectral index content. Acta Ecol. Sin. 2014, 34, 5736–5745. [Google Scholar]
- Blackburn, G.A. Quantifying chlorophylls and carotenoids at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Maccioni, A.; Agati, G.; Mazzinghi, P. New vegetation indices for remote measurement of chlorophylls based on leaf directional reflectance spectra. J. Photochem. Photobiol. B. 2001, 61, 52–61. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves. Spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Le Maire, G.; Francois, C.; Soudani, K.; Berveiller, D.; Pontailler, J.Y.; Breda, N.; Genet, H.; Davi, H.; Dufrene, E. Calibration and validation of hyperspectral indices for the estimation of broadleaved forest leaf chlorophyll content, leaf mass per area, leaf area index and leaf canopy biomass. Remote Sens. Environ. 2008, 112, 3846–3864. [Google Scholar] [CrossRef]
- Guan, L.; Liu, X. Hyperspectral recognition models for physiological ecology characterization of rice in Cd pollution stress. Ecol. Environ. Sci. 2009, 18, 488–493. [Google Scholar]
- Qi, J.; Chehbouni, A.; Huete, A.R.; Kerr, Y.H.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8(2), 127–150. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I. Principal Component Analysis, 2nd ed.; Springer: New York, NY, USA, 2002; pp. 1–9. [Google Scholar]
- Heim, R.H.-J.; Jürgens, N.; Große-Stoltenberg, A.; Oldeland, J. The effect of epidermal structures on leaf spectral signatures of ice plants (Aizoaceae). Remote Sens. 2015, 7, 16901–16914. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. 2018. R Package Version 0.1.7. Available online: https://CRAN.R project.org/package=ggpubr (accessed on 30 September 2019).
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll content in slash pine. Tree Physiol. 1990, 7, 33–48. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Red edge shift and biochemical content in grass canopies. ISPRS J. Photogramm. Remote Sens. 2007, 62, 34–42. [Google Scholar] [CrossRef]
- Le Maire, G.; François, C.; Dufrêne, E. Towards universal deciduous broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Vogelman, T.C.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Schwanninger, M.; Stefke, B.; Hinterstoisser, B. Qualitative assessment of acetylated wood with infrared spectroscopic methods. J. Near Infrared Spectrosc. 2011, 19, 349. [Google Scholar] [CrossRef]
- Serbin, S.P.; Dillaway, D.N.; Kruger, E.L.; Townsend, P.A. Leaf optical properties reflect variation in photosynthetic metabolism and its sensitivity to temperature. J. Exp. Bot. 2011, 63, 489–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamon, J.A.; Somers, B.; Malenovsky, Z.; Middleton, E.M.; Rascher, U.; Schaepman, M.E. Assessing Vegetation Function with Imaging Spectroscopy. Surv. Geophys. 2019, 40, 489–513. [Google Scholar] [CrossRef] [Green Version]
- Serbin, S.P.; Singh, A.; Mcneil, B.E.; Kingdon, C.C.; Townsend, P.A. Spectroscopic determination of leaf morphological and biochemical traits for northern temperate and boreal tree species. Ecol. Appl. 2014, 24, 1651–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, J.B. Introduction to Remote Sensing, 3rd ed.; Taylor and Francis: New York, NY, USA, 2002; pp. 1–9. [Google Scholar]
- Kubiske, M.E.; Pregitzer, K.S. Ecophysiological responses to simulated canopy gaps of two tree species of contrasting shade tolerance in CO2. Funct. Ecol. 1997, 11, 24–32. [Google Scholar] [CrossRef]
- Gamon, J.A.; Berry, J.A. Facultative and constitutive pigment effects on the Photochemical Reflectance Index (PRI) in sun and shade conifer needles. ISRJ Plant Sci. 2012, 60, 85–95. [Google Scholar] [CrossRef]









| Index | Formula | Reference |
|---|---|---|
| Carter | R550 | Carter and Knapp,2001 [48] |
| D2 | D720 | Miller et al., 1990 [49] |
| D1 | D703 | Boochs et al., 1990 [50] |
| Gitel1 | 1/R700 | Gitelson et al., 1999 [51] |
| SR1 | R750/R700 | Datt, 1998 [52] |
| SR2 | R750/R550 | Datt, 1998 [52] |
| SR3 | R675/R700 | Chappelle et al., 1992 [53] |
| SR4 | R695/R670 | Carter, 1994 [31] |
| SR5 | R710/R760 | Carter, 1994 [31] |
| SR6 | R695/R760 | Carter, 1994 [31] |
| SR7 | R605/R760 | Carter, 1994 [31] |
| SR8 | R800/R680 | Birth and McVey, 1968 [54] |
| SR9 | R672/R550 | Datt 1998 [52] |
| SR10 | R850/R710 | Datt, 1999 [55] |
| Datt1 | R860/(R550*R708) | Datt,1998 [52] |
| Datt2 | R672/(R550*R708) | Datt,1998 [52] |
| C1 | R675*R690/R2683 | Zarco-Tejada et al., 2009 [56] |
| NDVI1 | (R565-R735)/(R565+R735) | Gong et al., 2014 [57] |
| NDVI2 | (R800-R680)/(R800+R680) | Blackburn, 1998 [58] |
| NDVI3 | (R780-R710)/(R780-R680) | Maccioni et al., 2001 [59] |
| ND14 | (R850-R710)/(R850-R680) | Datt, 1999 [55] |
| NDVI 705, 750 | (R750-R705)/(R750+R705) | Gitelson and Merzlyak, 1994 [60] |
| Gitel | (R750-R800)/(R695-R740)-1 | Gitelson et al., 2003 [61] |
| DDn | 2*(R710-R(710-50)-R(710+50) | le Maire et al.,2008 [62] |
| MCARI1 | 1.5[1.2(R712-R670)-0.5(R712-R550)](R712/R670) | Guan and Liu, 2009 [63] |
| MSAVI | 0.5*(2*R800+1-SQRT((2*R800+1)2-8*(R800-R670))) | Qi et al., 1994 [64] |
| MCARI2 | ((R750-R705)-0.2*(R750-R550))*(R750/R705) | Wu et al., 2008 [65] |
| OSAVI1 | (1+0.16)*(R750-R705)/(R750+R705+0.16) | Wu et al., 2008 [65] |
| MCARI1/MSAVI | MCARI1/MSAVI | Guan and Liu, 2009 [63] |
| MCARI2/MSAVI | MCARI2/MSAVI | Wu et al., 2008 [65] |
| NDVI 680, 780 | ((R780-R680)/(R780+R680)) | Tucker, 1979 [66] |
| PRI 531, 570 | ((R531-R570)/(R531+R570)) | Gamon and Bond, 2013 [36] |
| Index | R2 Total | R2 Upper Canopy | R2 Lower Canopy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tree1 | Tree2 | Tree3 | Tree1 | Tree2 | Tree3 | Tree1 | Tree2 | Tree3 | |
| Carter | 0.430 | 0.278 | 0.438 | 0.550 | 0.562 | 0.369 | 0.243 | 0.065 | 0.089 |
| D2 | 0.436 | 0.299 | 0.508 | 0.610 | 0.717 | 0.389 | 0.175 | 0.037 | 0.187 |
| D1 | 0.425 | 0.271 | 0.511 | 0.569 | 0.647 | 0.398 | 0.207 | 0.062 | 0.056 |
| Gitel1 | 0.414 | 0.279 | 0.428 | 0.522 | 0.617 | 0.350 | 0.198 | 0.066 | 0.083 |
| SR1 | 0.437 | 0.298 | 0.455 | 0.568 | 0.671 | 0.370 | 0.195 | 0.059 | 0.106 |
| SR2 | 0.455 | 0.313 | 0.468 | 0.602 | 0.627 | 0.396 | 0.218 | 0.060 | 0.095 |
| SR3 | 0.437 | 0.292 | 0.485 | 0.538 | 0.636 | 0.281 | 0.191 | 0.060 | 0.063 |
| SR4 | 0.439 | 0.278 | 0.478 | 0.53 | 0.639 | 0.279 | 0.222 | 0.066 | 0.086 |
| SR5 | 0.452 | 0.296 | 0.494 | 0.612 | 0.715 | 0.404 | 0.212 | 0.056 | 0.120 |
| SR6 | 0.411 | 0.267 | 0.363 | 0.500 | 0.55 | 0.284 | 0.217 | 0.06 | 0.128 |
| SR7 | 0.394 | 0.242 | 0.356 | 0.493 | 0.411 | 0.271 | 0.22 | 0.047 | 0.120 |
| SR8 | 0.008 | 0.005 | 0.001 | 0.009 | 0.031 | 0.025 | 0.007 | 0.004 | 0.029 |
| SR9 | 0.459 | 0.310 | 0.472 | 0.595 | 0.649 | 0.261 | 0.212 | 0.063 | 0.047 |
| SR10 | 0.458 | 0.310 | 0.506 | 0.615 | 0.737 | 0.421 | 0.202 | 0.056 | 0.104 |
| Datt1 | 0.446 | 0.308 | 0.476 | 0.588 | 0.67 | 0.403 | 0.201 | 0.062 | 0.08 |
| Datt2 | 0.458 | 0.313 | 0.529 | 0.616 | 0.699 | 0.377 | 0.201 | 0.064 | 0.072 |
| C1 | 0.428 | 0.254 | 0.482 | 0.562 | 0.604 | 0.245 | 0.220 | 0.055 | 0.071 |
| NDVI1 | 0.441 | 0.290 | 0.469 | 0.585 | 0.563 | 0.390 | 0.225 | 0.056 | 0.113 |
| NDVI2 | 0.009 | 0.005 | 0.001 | 0.013 | 0.030 | 0.021 | 0.008 | 0.003 | 0.036 |
| NDVI3 | 0.457 | 0.299 | 0.518 | 0.625 | 0.733 | 0.405 | 0.214 | 0.057 | 0.112 |
| ND14 | 0.460 | 0.301 | 0.521 | 0.620 | 0.740 | 0.408 | 0.218 | 0.057 | 0.110 |
| NDVI 705, 750 | 0.448 | 0.296 | 0.486 | 0.599 | 0.701 | 0.397 | 0.208 | 0.057 | 0.119 |
| Gitel | 0.342 | 0.268 | 0.261 | 0.292 | 0.526 | 0.184 | 0.187 | 0.067 | 0.006 |
| DDn | 0.465 | 0.306 | 0.518 | 0.638 | 0.754 | 0.418 | 0.213 | 0.055 | 0.125 |
| MCARI1 | 0.434 | 0.272 | 0.484 | 0.541 | 0.617 | 0.298 | 0.224 | 0.064 | 0.058 |
| MSAVI | 0.178 | 0.021 | 0.001 | 0.008 | 0.110 | 0.023 | 0.001 | 0.022 | 0.068 |
| MCARI2 | 0.458 | 0.311 | 0.500 | 0.62 | 0.736 | 0.411 | 0.19 | 0.049 | 0.128 |
| OSAVI1 | 0.450 | 0.300 | 0.491 | 0.609 | 0.713 | 0.401 | 0.207 | 0.055 | 0.128 |
| MCARI1/MSAVI | 0.449 | 0.284 | 0.501 | 0.584 | 0.670 | 0.331 | 0.226 | 0.062 | 0.074 |
| MCARI2/MSAVI | 0.457 | 0.312 | 0.504 | 0.62 | 0.745 | 0.410 | 0.182 | 0.045 | 0.139 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deepak, M.; Keski-Saari, S.; Fauch, L.; Granlund, L.; Oksanen, E.; Keinänen, M. Leaf Canopy Layers Affect Spectral Reflectance in Silver Birch. Remote Sens. 2019, 11, 2884. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11242884
Deepak M, Keski-Saari S, Fauch L, Granlund L, Oksanen E, Keinänen M. Leaf Canopy Layers Affect Spectral Reflectance in Silver Birch. Remote Sensing. 2019; 11(24):2884. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11242884
Chicago/Turabian StyleDeepak, Maya, Sarita Keski-Saari, Laure Fauch, Lars Granlund, Elina Oksanen, and Markku Keinänen. 2019. "Leaf Canopy Layers Affect Spectral Reflectance in Silver Birch" Remote Sensing 11, no. 24: 2884. https://0-doi-org.brum.beds.ac.uk/10.3390/rs11242884