Soil Color and Mineralogy Mapping Using Proximal and Remote Sensing in Midwest Brazil
Abstract
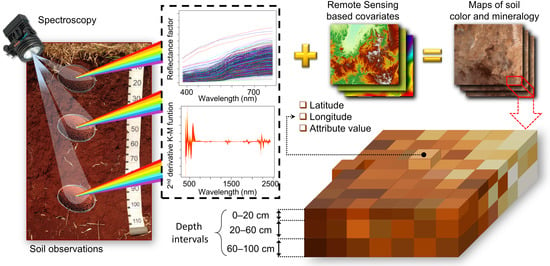
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Data
2.2. Reflectance to Soil Color
2.3. Reflectance to Soil Mineralogy
2.3.1. Spectral Processing
2.3.2. Key Spectral Bands for Mineral Quantification
2.4. Environmental Covariates
2.5. Soil Modelling by Random Forest (RF)
2.5.1. Model Tuning
2.5.2. Model Performance
2.5.3. Covariates’ Importance
2.5.4. Soil Mapping
3. Results
3.1. Soil Attributes Derived from Spectra
3.2. Performance of Spatial Models
3.3. Relevance of Covariates
3.4. Digital Maps of the Soil Surface and Subsurface
3.4.1. Gridded Munsell Soil Color
3.4.2. Spatial Patterns of the Main Minerals in Studied Soils
4. Discussion
4.1. Relationships Between Soil Color and Mineralogy
4.2. Use of Regression Models for Mapping Soil Properties
4.3. Influence of Environmental Predictors in Soil Color and Mineralogy Patterns
4.4. Comparison with Legacy Data and Maps
5. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Schwertmann, U.; Taylor, R.M. Iron Oxides. In Minerals in Soil Environments; Soil Science Society of America: Madison, WI, USA, 1989; pp. 379–438. [Google Scholar]
- Aitkenhead, M.J.; Coull, M.; Towers, W.; Hudson, G.; Black, H.I.J. Prediction of soil characteristics and colour using data from the National Soils Inventory of Scotland. Geoderma 2013, 200–201, 99–107. [Google Scholar] [CrossRef]
- Schwertmann, J.M. Relations Between Iron Oxides, Soil Color, and Soil Formation. In Soil Color; Ciolkosz, E.J., Bigham, U., Eds.; Soil Science Society of America: Madison, WI, USA, 1993; pp. 51–69. [Google Scholar]
- Schulze, D.G.; Nagel, J.L.; Van-Scoyoc, G.E.; Henderson, T.L.; Baumgardner, M.F.; Stott, D.E. Significance of Organic Matter in Determining Soil Colors. In Soil Color; Bigham, J.M., CiolKosz, E.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1993; pp. 71–90. [Google Scholar]
- Curi, N.; Franzmeier, D.P. Toposequence of Oxisols from the Central Plateau of Brazil. Soil Sci. Soc. Am. J. 1984, 48, 341–346. [Google Scholar] [CrossRef]
- Moraes, J.M. Geodiversidade do Estado de Goiás e do Distrito Federal; CPRM: Goiânia, Brazil, 2014. [Google Scholar]
- Schaefer, C.E.G.R.; Fabris, J.D.; Ker, J.C. Minerals in the clay fraction of Brazilian Latosols (Oxisols): A review. Clay Miner. 2008, 43, 137–154. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. Laboratory Measurement of Soil Color: Theory and Practice. In Soil Color; Soil Science Society of America: Madison, WI, USA, 1993; pp. 21–33. [Google Scholar]
- Barrón, V.; Torrent, J. Use of the Kubelka—Munk Theory to Study the Influence of Iron Oxides on Soil Colour. J. Soil Sci. 1986, 37, 499–510. [Google Scholar] [CrossRef]
- Silva, L.S.; Marques Júnior, J.; Barrón, V.; Gomes, R.P.; Teixeira, D.D.B.; Siqueira, D.S.; Vasconcelos, V. Spatial variability of iron oxides in soils from Brazilian sandstone and basalt. Catena 2020, 185, 104258. [Google Scholar] [CrossRef]
- Barrera-Bassols, N.; Alfred Zinck, J.; Van Ranst, E. Symbolism, knowledge and management of soil and land resources in indigenous communities: Ethnopedology at global, regional and local scales. Catena 2006, 65, 118–137. [Google Scholar] [CrossRef]
- Nawar, S.; Corstanje, R.; Halcro, G.; Mulla, D.; Mouazen, A.M. Delineation of Soil Management Zones for Variable-Rate Fertilization: A Review. In Advances in Agronomy; Sparks, D.L.B.T.-A., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 143, pp. 175–245. [Google Scholar]
- Embrapa—Brazilian Agricultural Research Corporation; National Soils Research Center. Brazilian Soil Classification System, 5th ed.; Embrapa-Cnps: Brasilia, Brazil, 2018; Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1094001/brazilian-soil-classification-system (accessed on 10 January 2020).
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014: International Soil Classification System for NAming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization: Rome, Italy, 2015; Available online: http://www.fao.org/3/i3794en/I3794EN.pdf (accessed on 10 January 2020).
- Hurst, V.J. Visual estimation of iron in saprolite. GSA Bull. 1977, 88, 174–176. [Google Scholar] [CrossRef]
- Munsell, A.H. A Color Notation; G. H. Ellis Company: Boston, MA, USA, 1907; Available online: http://books.google.com.br/books?id=PgcCAAAAYAAJ (accessed on 15 January 2020).
- Zhang, Y.; Hartemink, A.E. Digital mapping of a soil profile. Eur. J. Soil Sci. 2019, 70, 27–41. [Google Scholar] [CrossRef] [Green Version]
- Simon, T.; Zhang, Y.; Hartemink, A.E.; Huang, J.; Walter, C.; Yost, J.L. Predicting the color of sandy soils from Wisconsin, USA. Geoderma 2019, 361, 114039. [Google Scholar] [CrossRef]
- Marques, K.P.; Rizzo, R.; Dotto, A.C.; Souza, A.B.; Mello, F.A.; Neto, L.G.; Anjos, L.H.C.; Demattê, J.A. How qualitative spectral information can improve soil profile classification? J. Near Infrared Spectrosc. 2019. [Google Scholar] [CrossRef]
- Rizzo, R.; Demattê, J.A.M.; Lepsch, I.F.; Gallo, B.C.; Fongaro, C.T. Digital soil mapping at local scale using a multi-depth Vis–NIR spectral library and terrain attributes. Geoderma 2016, 274, 18–27. [Google Scholar] [CrossRef]
- Mattikalli, N.M. Soil color modeling for the visible and near-infrared bands of Landsat sensors using laboratory spectral measurements. Remote Sens. Environ. 1997, 59, 14–28. [Google Scholar] [CrossRef]
- Fernandez, R.N.; Schulze, D.G. Calculation of Soil Color from Reflectance Spectra. Soil Sci. Soc. Am. J. 1987, 51, 1277–1282. [Google Scholar] [CrossRef]
- Escadafal, R.; Girard, M.C.; Dominique, C. Modeling the relationships between Munsell soil color and soil spectral properties. Int. Agrophysics 1988, 4, 249–261. [Google Scholar]
- Scheinost, A.C.; Chavernas, A.; Barrón, V.; Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxide minerals in soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Bui, E.N.; de Caritat, P.; McKenzie, N.J. Mapping iron oxides and the color of Australian soil using visible–near-infrared reflectance spectra. J. Geophys. Res. 2010, 115, F04031. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Chen, C. Digitally mapping the information content of visible–near infrared spectra of surficial Australian soils. Remote Sens. Environ. 2011, 115, 1443–1455. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A. Fine-resolution multiscale mapping of clay minerals in Australian soils measured with near infrared spectra. J. Geophys. Res. Earth Surf. 2011, 116. [Google Scholar] [CrossRef]
- Malone, B.P.; Hughes, P.; McBratney, A.B.; Minasny, B. A model for the identification of terrons in the Lower Hunter Valley, Australia. Geoderma Reg. 2014, 1, 31–47. [Google Scholar] [CrossRef]
- Mulder, V.L.; Bruin, S.; Weyermann, J.; Kokaly, R.F.; Schaepman, M.E. Characterizing regional soil mineral composition using spectroscopy and geostatistics. Remote Sens. Environ. 2013, 139, 415–429. [Google Scholar] [CrossRef]
- Roberts, D.; Wilford, J.; Ghattas, O. Exposed soil and mineral map of the Australian continent revealing the land at its barest. Nat. Commun. 2019, 10, 5297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira Netto, J.S.; Bedidi, A.; Cervelle, B.; Pouget, M.; Flay, N. Visible spectrometric indices of hematite (Hm) and goethite (Gt) content in lateritic soils: The application of a Thematic Mapper (TM) image for soil-mapping in Brasilia, Brazil. Int. J. Remote Sens. 1997, 18, 2835–2852. [Google Scholar] [CrossRef]
- Ducart, D.F.; Silva, A.M.; Toledo, C.L.B.; Assis, L.M. de Mapping iron oxides with Landsat-8/OLI and EO-1/Hyperion imagery from the Serra Norte iron deposits in the Carajás Mineral Province, Brazil. Braz. J. Geol. 2016, 46, 331–349. [Google Scholar] [CrossRef]
- Samuel-Rosa, A.; Dalmolin, R.S.D.; Moura-Bueno, J.M.; Teixeira, W.G.; Alba, J.M.F. Open legacy soil survey data in Brazil: Geospatial data quality and how to improve it. Sci. Agric. 2020, 77, e20170430. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Cattle, S.R.; Ortega, A.; Fouad, Y. In situ measurements of soil colour, mineral composition and clay content by vis–NIR spectroscopy. Geoderma 2009, 150, 253–266. [Google Scholar] [CrossRef]
- Poppiel, R.R.; Lacerda, M.P.C.; Demattê, J.A.M.; Oliveira, M.P., Jr.; Gallo, B.C.; Safanelli, J.L. Pedology and soil class mapping from proximal and remote sensed data. Geoderma 2019, 328, 189–206. [Google Scholar] [CrossRef]
- de Mendes, W.S.; Medeiros Neto, L.G.; Demattê, J.A.M.; Gallo, B.C.; Rizzo, R.; Safanelli, J.L.; Fongaro, C.T. Is it possible to map subsurface soil attributes by satellite spectral transfer models? Geoderma 2019, 343, 269–279. [Google Scholar] [CrossRef]
- Poppiel, R.R.; Lacerda, M.P.C.; Oliveira, M.P., Jr.; Demattê, J.A.M.; Romero, D.J.; Sato, M.V.; Almeida, L.R., Jr.; Cassol, L.F.M. Surface Spectroscopy of Oxisols, Entisols and Inceptisol and Relationships with Selected Soil Properties. Revista Brasileira de Ciência do Solo 2018, 42, e0160519. [Google Scholar] [CrossRef] [Green Version]
- Rogge, D.; Bauer, A.; Zeidler, J.; Mueller, A.; Esch, T.; Heiden, U. Building an exposed soil composite processor (SCMaP) for mapping spatial and temporal characteristics of soils with Landsat imagery (1984–2014). Remote Sens. Environ. 2018, 205, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Demattê, J.A.M.; Fongaro, C.T.; Rizzo, R.; Safanelli, J.L. Geospatial Soil Sensing System (GEOS3): A powerful data mining procedure to retrieve soil spectral reflectance from satellite images. Remote Sens. Environ. 2018, 212, 161–175. [Google Scholar] [CrossRef]
- Poppiel, R.R.; Lacerda, P.C.M.; Safanelli, L.J.; Rizzo, R.; Oliveira, P.M.; Novais, J.J.; Demattê, A.M.J. Mapping at 30 m Resolution of Soil Attributes at Multiple Depths in Midwest Brazil. Remote Sens. 2019, 11, 2905. [Google Scholar] [CrossRef] [Green Version]
- Lagacherie, P. Digital Soil Mapping: A State of the Art. In Digital Soil Mapping with Limited Data; Hartemink, A., McBratney, A., Mendonça-Santos, M.L., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 3–14. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Scornet, E.; Biau, G.; Vert, J.-P. Consistency of random forests. Ann. Stat. 2015, 43, 1716–1741. [Google Scholar] [CrossRef]
- Vieira, B.C.; Salgado, A.A.R.; Santos, L.J.C. Landscapes and Landforms of Brazil; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- IBGE—Instituto Brasileiro de Geografia e Estatística Pedologia. Available online: https://www.ibge.gov.br/geociencias/informacoes-ambientais/pedologia/10871-pedologia.html?=&t=downloads (accessed on 30 September 2019).
- Demattê, J.A.M.; Dotto, A.C.; Paiva, A.F.S.; Sato, M.V.; Dalmolin, R.S.D.; do Araújo, M.S.B.; Silva, E.B.; Nanni, M.R.; ten Caten, A.; Noronha, N.C.; et al. The Brazilian Soil Spectral Library (BSSL): A general view, application and challenges. Geoderma 2019, 354, 113793. [Google Scholar] [CrossRef]
- Canadell, J.; Jackson, R.B.; Ehleringer, J.B.; Mooney, H.A.; Sala, O.E.; Schulze, E.-D. Maximum rooting depth of vegetation types at the global scale. Oecologia 1996, 108, 583–595. [Google Scholar] [CrossRef]
- Stevens, A.; Ramirez-Lopez, L. Prospectr: Processing and Sample Selection for Vis-NIR Spectral Data. 2013. Available online: https://cran.r-project.org/package=prospectr (accessed on 18 December 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Wyszecki, G.; Stiles, W.S. Color Science: Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Centore, P. The Munsell and Kubelka-Munk Toolbox; GitHub: San Francisco, CA, USA, 2014; Available online: http://centore.isletech.net/~centore/MunsellAndKubelkaMunkToolbox/MunsellAndKubelkaMunkToolbox.html (accessed on 19 December 2019).
- Borchers, H.W. Pracma: Practical Numerical Math Functions. 2019. Available online: https://cran.r-project.org/package=pracma (accessed on 20 December 2019).
- Agostinelli, C. CircStats: Circular Statistics, from “Topics in Circular Statistics”. 2018. Available online: https://cran.r-project.org/package=CircStats (accessed on 20 December 2019).
- Torrent, J.; Barrón, V. Diffuse Reflectance Spectroscopy of Iron Oxides. Encycl. Surf. Colloid Sci. 2002, 1, 1438–1446. [Google Scholar]
- CAMO Software Inc. The Unscrambler Version 9.7; CAMO Software AS: Woodbridge, NJ, USA, 2007. [Google Scholar]
- Kosmas, C.S.; Curi, N.; Bryant, R.B.; Franzmeier, D.P. Characterization of Iron Oxide Minerals by Second-Derivative Visible Spectroscopy. Soil Sci. Soc. Am. J. 1984, 48, 401–405. [Google Scholar] [CrossRef]
- Macedo, J.; Bryant, R.B. Morphology, Mineralogy, and Genesis of a Hydrosequence of Oxisols in Brazil. Soil Sci. Soc. Am. J. 1987, 51, 690–698. [Google Scholar] [CrossRef]
- Gomes, J.B.V.; Curi, N.; Schulze, D.G.; Marques, J.J.G.S.M.; Ker, J.C.; Motta, P.E.F. Mineralogia, morfologia e análise microscópica de solos do bioma cerrado. Revista Brasileira de Ciência do Solo 2004, 28, 679–694. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Lal, R.; Bigham, J.M.; Resck, D.V.S. Edaphic Controls on Soil Organic Carbon Retention in the Brazilian Cerrado: Texture and Mineralogy. Soil Sci. Soc. Am. J. 2007, 71, 1204–1214. [Google Scholar] [CrossRef]
- Terra, F.S.; Demattê, J.A.M.; Viscarra Rossel, R.A. Proximal spectral sensing in pedological assessments: Vis–NIR spectra for soil classification based on weathering and pedogenesis. Geoderma 2018, 318, 123–136. [Google Scholar] [CrossRef]
- Hamilton, N. ggtern: An Extension to “ggplot2”, for the Creation of Ternary Diagrams. J. Stat. Softw. 2018, 87, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.N.; King, T.V.V.; Klejwa, M.; Swayze, G.A.; Vergo, N. High spectral resolution reflectance spectroscopy of minerals. J. Geophys. Res. Solid Earth 1990, 95, 12653–12680. [Google Scholar] [CrossRef] [Green Version]
- McBratney, A.B.; Mendonça Santos, M.L.; Minasny, B. On digital soil mapping. Geoderma 2003, 117, 3–52. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- CPRM—Companhia de Pesquisa de Recursos Minerais. Carta Geológica do Brasil ao Milionésimo: Sistema de Informações Geográficas-SIG; CPRM: Brasília, Brazil, 2004. Available online: http://www.cprm.gov.br/publique/Geologia/Geologia-Basica/Carta-Geologica-do-Brasil-ao-Milionesimo-298.html (accessed on 15 January 2020).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Tadono, T.; Ishida, H.; Oda, F.; Naito, S.; Minakawa, K.; Iwamoto, H. Precise global DEM generation by ALOS PRISM. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, 2, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Hengl, T.; de Jesus, J.M.; MacMillan, R.A.; Batjes, N.H.; Heuvelink, G.B.M.; Ribeiro, E.; Samuel-Rosa, A.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; et al. SoilGrids1km—Global Soil Information Based on Automated Mapping. PLoS ONE 2014, 9, e105992. [Google Scholar] [CrossRef] [Green Version]
- Hengl, T.; Heuvelink, G.B.M.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; Shepherd, K.D.; Sila, A.; MacMillan, R.A.; Mendes de Jesus, J.; Tamene, L.; et al. Mapping Soil Properties of Africa at 250 m Resolution: Random Forests Significantly Improve Current Predictions. PLoS ONE 2015, 10, e0125814. [Google Scholar] [CrossRef]
- Gomes, L.C.; Faria, R.M.; de Souza, E.; Veloso, G.V.; Schaefer, C.E.G.R.; Filho, E.I.F. Modelling and mapping soil organic carbon stocks in Brazil. Geoderma 2019, 340, 337–350. [Google Scholar] [CrossRef]
- Hengl, T.; Mendes de Jesus, J.; Heuvelink, G.B.M.; Ruiperez Gonzalez, M.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loiseau, T.; Chen, S.; Mulder, V.L.; Román Dobarco, M.; Richer-de-Forges, A.C.; Lehmann, S.; Bourennane, H.; Saby, N.P.A.; Martin, M.P.; Vaudour, E.; et al. Satellite data integration for soil clay content modelling at a national scale. Int. J. Appl. Earth Obs. Geoinf. 2019, 82, 101905. [Google Scholar] [CrossRef]
- Wadoux, A.M.-C.; Brus, D.J.; Heuvelink, G.B.M. Sampling design optimization for soil mapping with random forest. Geoderma 2019, 355, 113913. [Google Scholar] [CrossRef]
- Leenaars, J.G.B.; Elias, E.; Wösten, J.H.M.; Ruiperez-González, M.; Kempen, B. Mapping the major soil-landscape resources of the Ethiopian Highlands using random forest. Geoderma 2019, 361, 114067. [Google Scholar] [CrossRef]
- Silva, B.P.C.; Silva, M.L.N.; Avalos, F.A.P.; de Menezes, M.D.; Curi, N. Digital soil mapping including additional point sampling in Posses ecosystem services pilot watershed, southeastern Brazil. Sci. Rep. 2019, 9, 13763. [Google Scholar] [CrossRef] [Green Version]
- Hengl, T.; Nussbaum, M.; Wright, M.N.; Heuvelink, G.B.M.; Gräler, B. Random forest as a generic framework for predictive modeling of spatial and spatio-temporal variables. PeerJ 2018, 6, e5518. [Google Scholar] [CrossRef] [Green Version]
- Wright, M.N.; Ziegler, A. Ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. arXiv 2015, arXiv:1508.04409. [Google Scholar] [CrossRef] [Green Version]
- Probst, P.; Wright, M.N.; Boulesteix, A.-L. Hyperparameters and tuning strategies for random forest. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2019, 9, e1301. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M. Caret: Classification and Regression Training. 2019. Available online: https://cran.r-project.org/web/packages/caret/index.html (accessed on 15 December 2019).
- Padarian, J.; Minasny, B.; McBratney, A.B. Machine learning and soil sciences: A review aided by machine learning tools. SOIL Discuss. 2019, 2019, 1–29. [Google Scholar] [CrossRef] [Green Version]
- FAO. Soil Organic Carbon Mapping Cookbook, 2nd ed.; FAO: Rome, Italy, 2018. [Google Scholar]
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.-M.; McBratney, A. Critical review of chemometric indicators commonly used for assessing the quality of the prediction of soil attributes by NIR spectroscopy. TrAC Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Fernandez, R.N.; Schulze, D.G. Munsell Colors of Soils Simulated by Mixtures of Goethite and Hematite with Kaolinite. Zeitschrift für Pflanzenernährung und Bodenkunde 1992, 155, 473–478. [Google Scholar] [CrossRef]
- Zinn, Y.L.; Bigham, J.M. Pedogenic and lithogenic gravels as indicators of soil polygenesis in the Brazilian Cerrado. Soil Res. 2016, 54, 440–450. [Google Scholar] [CrossRef]
- Barbosa, I.O.; Lacerda, M.P.C.; Bilich, M.R. Pedomorphogeological relations in the chapadas elevadas of the Distrito Federal, Brazil. Revista Brasileira de Ciência do Solo 2009, 33, 1373–1383. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.E. Mineralogy and Genesis of a Sequence of Cerrados Soils in the Federal District. Master’s Thesis, University of Rio Grande do Sul, Porto Alegre, Brzsil, 1977. Available online: https://www.ufrgs.br/agronomia/materiais/19777dt.pdf (accessed on 20 January 2020).
- Melo, V.F.; Singh, B.; Schaefer, C.E.G.R.; Novais, R.F.; Fontes, M.P.F. Chemical and Mineralogical Properties of Kaolinite-Rich Brazilian Soils. Soil Sci. Soc. Am. J. 2001, 65, 1324–1333. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.-L.; Song, X.; Li, D.; Zhao, Y.; Yang, J.; Wu, H.; Yang, F. High-resolution and three-dimensional mapping of soil texture of China. Geoderma 2020, 361, 114061. [Google Scholar] [CrossRef]
- Hengl, T.; MacMillan, R.A. Predictive Soil Mapping with R; OpenGeoHub Foundation: Wageningen, The Netherlands, 2019. [Google Scholar]
- Liles, G.C.; Beaudette, D.E.; O’Geen, A.T.; Horwath, W.R. Developing predictive soil C models for soils using quantitative color measurements. Soil Sci. Soc. Am. J. 2013, 77, 2173–2181. [Google Scholar] [CrossRef]
- Miller, B.A.; Koszinski, S.; Wehrhan, M.; Sommer, M. Impact of multi-scale predictor selection for modeling soil properties. Geoderma 2015, 239–240, 97–106. [Google Scholar] [CrossRef]
- Post, D.F.; Lucas, W.M.; White, S.A.; Ehasz, M.J.; Batchily, A.K.; Horvath, E.H. Relations between Soil Color and Landsat Reflectance on Semiarid Rangelands. Soil Sci. Soc. Am. J. 1994, 58, 1809–1816. [Google Scholar] [CrossRef]
- Liu, F.; Geng, X.; Zhu, A.-X.; Fraser, W.; Waddell, A. Soil texture mapping over low relief areas using land surface feedback dynamic patterns extracted from MODIS. Geoderma 2012, 171–172, 44–52. [Google Scholar] [CrossRef]
- Maynard, J.J.; Levi, M.R. Hyper-temporal remote sensing for digital soil mapping: Characterizing soil-vegetation response to climatic variability. Geoderma 2017, 285, 94–109. [Google Scholar] [CrossRef] [Green Version]
- Das, S. Comparison among influencing factor, frequency ratio, and analytical hierarchy process techniques for groundwater potential zonation in Vaitarna basin, Maharashtra, India. Groundw. Sustain. Dev. 2019, 8, 617–629. [Google Scholar] [CrossRef]
- Reatto, A.; Bruand, A.; de Souza Martins, E.; Muller, F.; da Silva, E.M.; de Carvalho, O.A.; Brossard, M. Variation of the kaolinite and gibbsite content at regional and local scale in Latosols of the Brazilian Central Plateau. C. R. Geosci. 2008, 340, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Ramcharan, A.; Hengl, T.; Nauman, T.; Brungard, C.; Waltman, S.; Wills, S.; Thompson, J. Soil Property and Class Maps of the Conterminous United States at 100-Meter Spatial Resolution. Soil Sci. Soc. Am. J. 2018, 82, 186–201. [Google Scholar] [CrossRef] [Green Version]








| Soil Mineral | Minima Band Position (nm) | Maxima Band Position (nm) | Band Amplitude | Reference for Band Positions |
|---|---|---|---|---|
| Goethite | ~415* | ~455* | AGt | [24,56] |
| Hematite | 535* | 580* | AHt | [24] |
| 2:1 clay minerals1 | 1900–1925 | 1870–1895 | A2:1 | [62] |
| Kaolinite | 2205 | 2225 | AKt | [62] |
| Gibbsite | 2265 | 2295 | AGb | [62] |
| Factor | Covariate | Description |
|---|---|---|
| Soil, Parent Material and Age | SySI | Synthetic Soil Image based Landsat 4, 5, 7 and 8 (7 bands), representing bare soil reflectance at 30 m resolution. |
| Geological Lineaments | Meters of structural features per km2 from CPRM data at 1:1,000,000 scale [65]. | |
| Organisms | SyVIw and SyVId | Synthetic Vegetation Image of wet (Nov-Mar) and dry (May-Sep) seasons based Landsat 4 and 5 (7 bands), representing potential natural vegetation reflectance at 30 m resolution. |
| Climate | Annual Precipitation (mm) | Bioclimatic variables obtained from the WorldClim dataset at 1 km resolution [66]. |
| Precipitation Seasonality (CV) | ||
| Annual Mean Temperature (°C) | ||
| Temperature Annual Range (°C) | ||
| Temperature Seasonality (°C) | ||
| Relief | Elevation (m) | Terrain attributes obtained from the 30 m ALOS digital elevation model [67] |
| Slope (degree) | ||
| Aspect (degree) | ||
| Topographic Position Index (m) | ||
| Horizontal Curvature (m) | ||
| Vertical Curvature (m) |
| Soil Attribute | Depth2 | mTry | minNS | RMSEcal | RPIQcal | R2cal | RMSE10cv | RPIQ10cv | R210cv |
|---|---|---|---|---|---|---|---|---|---|
| Hue number1 | 0–20 | 24 | 5 | 0.53 | 5.89 | 0.93 | 1.30 | 2.35 | 0.58 |
| 20–60 | 24 | 5 | 0.61 | 5.45 | 0.93 | 1.50 | 2.17 | 0.54 | |
| 60–100 | 33 | 5 | 0.56 | 4.80 | 0.92 | 1.40 | 1.91 | 0.50 | |
| Value | 0–20 | 24 | 5 | 0.19 | 4.84 | 0.93 | 0.50 | 1.95 | 0.59 |
| 20–60 | 24 | 5 | 0.24 | 5.85 | 0.92 | 0.60 | 2.44 | 0.55 | |
| 60–100 | 24 | 5 | 0.21 | 5.79 | 0.94 | 0.50 | 2.32 | 0.64 | |
| Chroma | 0–20 | 33 | 5 | 0.27 | 3.67 | 0.89 | 0.70 | 1.45 | 0.31 |
| 20–60 | 33 | 5 | 0.31 | 3.56 | 0.88 | 0.80 | 1.41 | 0.24 | |
| 60–100 | 33 | 5 | 0.29 | 3.41 | 0.90 | 0.70 | 1.36 | 0.38 | |
| Goethite | 0–20 | 24 | 5 | 414* | 4.07 | 0.91 | 1008* | 1.67 | 0.45 |
| 20–60 | 24 | 5 | 396* | 4.06 | 0.91 | 990* | 1.62 | 0.45 | |
| 60–100 | 6 | 5 | 494* | 2.97 | 0.85 | 1122* | 1.31 | 0.24 | |
| Hematite | 0–20 | 24 | 5 | 436* | 6.49 | 0.96 | 1102* | 2.57 | 0.71 |
| 20–60 | 24 | 5 | 496* | 6.49 | 0.96 | 1254* | 2.57 | 0.72 | |
| 60–100 | 24 | 5 | 504* | 6.46 | 0.96 | 1264* | 2.58 | 0.72 | |
| Kaolinite | 0–20 | 33 | 5 | 171* | 4.41 | 0.91 | 424* | 1.78 | 0.47 |
| 20–60 | 33 | 5 | 205* | 4.86 | 0.93 | 508* | 1.96 | 0.55 | |
| 60–100 | 33 | 5 | 190* | 5.20 | 0.94 | 481* | 2.05 | 0.59 | |
| Gibbsite | 0–20 | 24 | 5 | 123* | 3.23 | 0.93 | 309* | 1.28 | 0.55 |
| 20–60 | 33 | 5 | 132* | 3.55 | 0.94 | 335* | 1.40 | 0.64 | |
| 60–100 | 24 | 5 | 124* | 4.09 | 0.95 | 312* | 1.62 | 0.65 | |
| 2:1 minerals | 0–20 | 33 | 5 | 54* | 3.99 | 0.90 | 132* | 1.63 | 0.43 |
| 20–60 | 24 | 5 | 56* | 3.75 | 0.92 | 139* | 1.52 | 0.51 | |
| 60–100 | 24 | 5 | 63* | 3.13 | 0.91 | 151* | 1.31 | 0.49 |
| Depth | Hue < 2.5YR | 2.5YR ≤ Hue < 5YR | 5YR ≤ Hue ≤ 7.5YR | 7.5YR < Hue |
|---|---|---|---|---|
| (cm) | Area (%) | |||
| 0–20 | 1 | 27 | 66 | 6 |
| 20–60 | 4 | 49 | 46 | 1 |
| 60–100 | 16 | 49 | 23 | 13 |
| Average | 7 | 42 | 45 | 7 |
| Relative Amount1 | 0−20 cm | 20−60 cm | 60−100 cm | Average |
|---|---|---|---|---|
| (%) | Area (%) | |||
| 9 < Ht ≤ 31 | 51 | 48 | 42 | 47 |
| 31 < Ht ≤ 49 | 36 | 46 | 52 | 45 |
| 49 < Ht ≤ 66 | 13 | 5 | 6 | 8 |
| 6 < Gt ≤ 24 | 23 | 12 | 6 | 14 |
| 24 < Gt ≤ 37 | 55 | 69 | 72 | 65 |
| 37 < Gt ≤ 50 | 22 | 19 | 23 | 21 |
| 4 < Kt ≤ 19 | 46 | 44 | 43 | 44 |
| 19 < Kt ≤ 31 | 34 | 38 | 50 | 41 |
| 31 < Kt ≤ 50 | 21 | 17 | 7 | 15 |
| 1 < Gb ≤ 9 | 69 | 63 | 60 | 64 |
| 9 < Gb ≤ 17 | 26 | 20 | 38 | 28 |
| 17 < Gb ≤ 29 | 5 | 17 | 2 | 8 |
| 2 < 2:1 ≤ 7 | 58 | 79 | 91 | 76 |
| 7 < 2:1 ≤ 13 | 34 | 20 | 9 | 21 |
| 13 < 2:1 ≤ 18 | 8 | 0.4 | 0 | 3 |
| Depth | Legacy Data | Our Predicted Maps | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (cm) | Total Elements1 | n | HueN | Value | Chroma | Ht | Gt | Gb | Kt | 2:1 | |
| 0–20 | Fe2O3 | 225 | −0.35 | −0.39 | 0.10 | 0.39 | −0.03 | 0.06 | 0.09 | 0.12 | |
| Al2O3 | 878 | −0.01 | −0.12 | 0.05 | 0.09 | 0.10 | 0.25 | 0.25 | 0.01 | ||
| TiO2 | 782 | −0.12 | −0.16 | 0.03 | 0.11 | 0.04 | 0.13 | 0.06 | 0.19 | ||
| 20–60 | Fe2O3 | 124 | −0.26 | −0.39 | −0.12 | 0.38 | 0.11 | 0.06 | 0.00 | 0.09 | |
| Al2O3 | 729 | −0.03 | 0.10 | 0.04 | 0.04 | 0.23 | 0.20 | 0.23 | 0.06 | ||
| TiO2 | 639 | −0.27 | −0.12 | −0.03 | 0.20 | 0.28 | 0.10 | 0.14 | 0.07 | ||
| 60–100 | Fe2O3 | 174 | −0.18 | −0.53 | −0.30 | 0.56 | 0.30 | 0.15 | 0.00 | 0.51 | |
| Al2O3 | 532 | −0.06 | −0.04 | −0.09 | 0.06 | 0.33 | 0.37 | 0.06 | 0.05 | ||
| TiO2 | 479 | −0.06 | −0.23 | −0.14 | 0.22 | 0.29 | 0.14 | −0.15 | 0.20 | ||
| Munsell color2 | |||||||||||
| 0–20 | Hue number | 230 | 0.53 | 0.38 | −0.24 | −0.39 | −0.26 | −0.01 | −0.06 | −0.10 | |
| Value | 230 | 0.32 | 0.37 | 0.01 | −0.24 | −0.07 | 0.06 | −0.07 | −0.18 | ||
| Chroma | 230 | 0.01 | 0.02 | 0.16 | 0.01 | 0.23 | 0.32 | 0.15 | −0.05 | ||
| 20–60 | Hue number | 195 | 0.63 | 0.44 | −0.11 | −0.40 | −0.30 | 0.02 | −0.11 | −0.02 | |
| Value | 195 | 0.48 | 0.46 | 0.03 | −0.35 | −0.32 | 0.04 | −0.10 | 0.04 | ||
| Chroma | 195 | 0.05 | 0.17 | 0.14 | −0.04 | 0.11 | 0.24 | 0.19 | 0.13 | ||
| 60–100 | Hue number | 143 | 0.35 | 0.46 | −0.16 | −0.44 | −0.15 | 0.05 | 0.12 | −0.06 | |
| Value | 143 | 0.42 | 0.58 | −0.23 | −0.46 | −0.38 | −0.06 | 0.09 | −0.11 | ||
| Chroma | 143 | −0.01 | 0.05 | 0.19 | −0.06 | 0.21 | 0.12 | 0.01 | −0.12 | ||
| Legacy soil map | |||||||||||
| 0–20 | Weather. degree3 | 5k* | −0.38 | −0.34 | 0.08 | 0.42 | 0.23 | 0.19 | 0.09 | −0.02 | |
| Hue number4 | 5k* | 0.48 | 0.47 | −0.10 | −0.52 | −0.27 | −0.17 | −0.05 | −0.13 | ||
| 20–60 | Weather. degree3 | 5k* | −0.27 | −0.35 | 0.03 | 0.40 | 0.35 | 0.17 | −0.02 | 0.10 | |
| Hue number4 | 5k* | 0.39 | 0.47 | 0.03 | −0.49 | −0.42 | −0.10 | −0.03 | −0.22 | ||
| 60–100 | Weather. degree3 | 5k* | −0.15 | −0.38 | −0.09 | 0.37 | 0.31 | 0.23 | −0.03 | 0.02 | |
| Hue number4 | 5k* | 0.29 | 0.48 | 0.20 | −0.47 | −0.38 | −0.18 | 0.00 | −0.17 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poppiel, R.R.; Lacerda, M.P.C.; Rizzo, R.; Safanelli, J.L.; Bonfatti, B.R.; Silvero, N.E.Q.; Demattê, J.A.M. Soil Color and Mineralogy Mapping Using Proximal and Remote Sensing in Midwest Brazil. Remote Sens. 2020, 12, 1197. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12071197
Poppiel RR, Lacerda MPC, Rizzo R, Safanelli JL, Bonfatti BR, Silvero NEQ, Demattê JAM. Soil Color and Mineralogy Mapping Using Proximal and Remote Sensing in Midwest Brazil. Remote Sensing. 2020; 12(7):1197. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12071197
Chicago/Turabian StylePoppiel, Raúl Roberto, Marilusa Pinto Coelho Lacerda, Rodnei Rizzo, José Lucas Safanelli, Benito Roberto Bonfatti, Nélida Elizabet Quiñonez Silvero, and José Alexandre Melo Demattê. 2020. "Soil Color and Mineralogy Mapping Using Proximal and Remote Sensing in Midwest Brazil" Remote Sensing 12, no. 7: 1197. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12071197