Seasonal Cycles of Phytoplankton Expressed by Sine Equations Using the Daily Climatology from Satellite-Retrieved Chlorophyll-a Concentration (1997–2019) Over Global Ocean
Abstract
:1. Introduction
2. Methodology and Data
2.1. The Sine Equation for Annual Cycle
2.2. The Sine Equation for the Semiannual Cycle
2.3. The Sine Equation for Multiple Cycles
2.4. The Mean Relative Difference
2.5. Data
3. Results and Discussion
3.1. The Parameters of the Nonlinear Fit Function
3.1.1. Mean Chl-a
3.1.2. Amplitudes of Different Cycles
3.1.3. Phases of Different Cycles
3.2. Comparison of Different Sine Equations
3.3. The Effects of Climatology on Seasonal Cycles
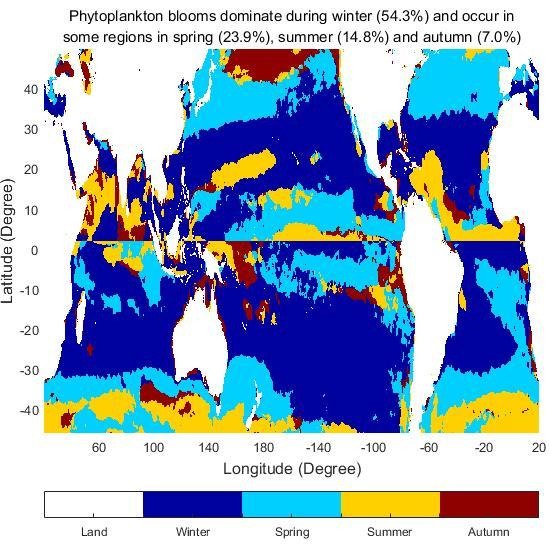
3.4. The Timing of Phytoplankton Blooms
3.5. The Effects of Equation (3) on Four Sinusoids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoder, J.A.; McClain, C.R.; Feldman, G.C.; Esaias, W.E. Annual cycles of phytoplankton chlorophyll concentrations in the global ocean: A satellite view. Glob. Biogeochem. Cycles 1993, 7, 181–193. [Google Scholar] [CrossRef]
- Moore, J.K.; Abbott, M.R. Phytoplankton chlorophyll distributions and primary production in the Southern Ocean. J. Geophys. Res. Space Phys. 2000, 105, 28709–28722. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; O’Malley, R.T.; Siegel, D.A.; McClain, C.R.; Sarmiento, J.L.; Feldman, G.C.; Milligan, A.J.; Falkowski, P.G.; Letelier, R.M.; Boss, E. Climate-driven trends in contemporary ocean productivity. Nature 2006, 444, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Vantrepotte, V.; Mélin, F. Inter-annual variations in the SeaWiFS global chlorophyll a concentration (1997–2007). Deep Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 429–441. [Google Scholar] [CrossRef]
- Cole, H.; Henson, S.A.; Martin, A.; Yool, A. Mind the gap: The impact of missing data on the calculation of phytoplankton phenology metrics. J. Geophys. Res. Space Phys. 2012, 117. [Google Scholar] [CrossRef]
- Salgado-Hernanz, P.; Racault, M.-F.; Font-Muñoz, J.; Basterretxea, G. Trends in phytoplankton phenology in the Mediterranean Sea based on ocean-colour remote sensing. Remote Sens. Environ. 2019, 221, 50–64. [Google Scholar] [CrossRef]
- Platt, T.; White, G.N.; Zhai, L.; Sathyendranath, S.; Roy, S. The phenology of phytoplankton blooms: Ecosystem indicators from remote sensing. Ecol. Model. 2009, 220, 3057–3069. [Google Scholar] [CrossRef]
- Cushing, D.H. The seasonal variation in oceanic production as a problem in population dynamics. ICES J. Mar. Sci. 1959, 24, 455–464. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Randerson, J.T.; McClain, C.R.; Feldman, G.C.; Los, S.O.; Tucker, C.J.; Falkowski, P.G.; Field, C.B.; Frouin, R.; Esaias, W.E.; et al. Biospheric Primary Production During an ENSO Transition. Science 2001, 291, 2594–2597. [Google Scholar] [CrossRef] [Green Version]
- Polovina, J.J.; Howell, E.; Kobayashi, D.R.; Seki, M.P. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 2001, 49, 469–483. [Google Scholar] [CrossRef]
- Henson, S.A.; Dunne, J.P.; Sarmiento, J.L. Decadal variability in North Atlantic phytoplankton blooms. J. Geophys. Res. Space Phys. 2009, 114. [Google Scholar] [CrossRef]
- Grodsky, S.A.; Carton, J.A.; McClain, C.R. Variability of upwelling and chlorophyll in the equatorial Atlantic. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef] [Green Version]
- Kahru, M.; Gille, S.T.; Murtugudde, R.; Strutton, P.G.; Sarabia, M.M.; Wang, H.; Mitchell, B.G. Global correlations between winds and ocean chlorophyll. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Navarro, G.; Caballero, I.; Prieto, L.; Vazquez, A.; Flecha, S.; Huertas, I.E.; Ruiz, J. Seasonal-to-interannual variability of chlorophyll-a bloom timing associated with physical forcing in the Gulf of Cádiz. Adv. Space Res. 2012, 50, 1164–1172. [Google Scholar] [CrossRef] [Green Version]
- Ardyna, M.; Claustre, H.; Sallée, J.-B.; D’Ovidio, F.; Gentili, B.; Van Dijken, G.L.; D’Ortenzio, F.; Arrigo, K.R. Delineating environmental control of phytoplankton biomass and phenology in the Southern Ocean. Geophys. Res. Lett. 2017, 44, 5016–5024. [Google Scholar] [CrossRef]
- Lan, K.-W.; Shimada, T.; Lee, M.-A.; Su, N.-J.; Chang, Y. Using Remote-Sensing Environmental and Fishery Data to Map Potential Yellowfin Tuna Habitats in the Tropical Pacific Ocean. Remote Sens. 2017, 9, 444. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Lan, K.-W.; Ho, H.-Y.; Wang, K.-Y.; Wu, Y.-L. Variation in the Catch Rate and Distribution of Swordtip Squid Uroteuthis edulis Associated with Factors of the Oceanic Environment in the Southern East China Sea. Mar. Coast. Fish. 2018, 10, 452–464. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Xing, X.; Liu, H.; Yuan, Y.; Wang, Y.; Chai, F. The variability of chlorophyll-a and its relationship with dynamic factors in the basin of the South China Sea. J. Mar. Syst. 2019, 200, 103230. [Google Scholar] [CrossRef]
- Yamada, K.; Ishizaka, J. Estimation of interdecadal change of spring bloom timing, in the case of the Japan Sea. Geophys. Res. Lett. 2006, 33, 02608. [Google Scholar] [CrossRef]
- Zhai, L.; Platt, T.; Tang, C.; Sathyendranath, S.; Hernandez-Walls, R. Phytoplankton phenology on the Scotian Shelf. ICES J. Mar. Sci. 2011, 68, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Vargas, M.; Brown, C.W.; Sapiano, M.R.P. Phenology of marine phytoplankton from satellite ocean color measurements. Geophys. Res. Lett. 2009, 36, 329–342. [Google Scholar] [CrossRef] [Green Version]
- Sapiano, M.R.P.; Brown, C.W.; Uz, S.S.; Vargas, M. Establishing a global climatology of marine phytoplankton phenological characteristics. J. Geophys. Res. Space Phys. 2012, 117. [Google Scholar] [CrossRef]
- Huang, N.E.; Wu, Z. A review on Hilbert-Huang transform: Method and its applications to geophysical studies. Rev. Geophys. 2008, 46. [Google Scholar] [CrossRef] [Green Version]
- Palacz, A.P.; Xue, H.; Armbrecht, C.; Zhang, C.; Chai, F. Seasonal and inter-annual changes in the surface chlorophyll of the South China Sea. J. Geophys. Res. Space Phys. 2011, 116. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Qiao, F.; Deng, J.; Wang, G. Shifting Trends in Bimodal Phytoplankton Blooms in the North Pacific and North Atlantic Oceans From Space With the Holo-Hilbert Spectral Analysis. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 57–64. [Google Scholar] [CrossRef]
- Wernand, M.R.; Van Der Woerd, H.J.; Gieskes, W.W.C. Trends in Ocean Colour and Chlorophyll Concentration from 1889 to 2000, Worldwide. PLoS ONE 2013, 8, e63766. [Google Scholar] [CrossRef]
- Winder, M.; Cloern, J.E. The annual cycles of phytoplankton biomass. Philos. Trans. R. Soc. B: Biol. Sci. 2010, 365, 3215–3226. [Google Scholar] [CrossRef]
- Wang, Y.; Castelao, R.M.; Yuan, Y. Seasonal variability of alongshore winds and sea surface temperature fronts in Eastern Boundary Current Systems. J. Geophys. Res. Oceans 2015, 120, 2385–2400. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Shu, Q.; Zhao, C.; Wang, G.; Wu, Z.; Qiao, F. Spatiotemporal evolution of the chlorophyll a trend in the North Atlantic Ocean. Sci. Total Environ. 2018, 612, 1141–1148. [Google Scholar] [CrossRef]
- Boyce, D.G.; Petrie, B.; Frank, K.T.; Worm, B.; Leggett, W.C. Environmental structuring of marine plankton phenology. Nat. Ecol. Evol. 2017, 1, 1484–1494. [Google Scholar] [CrossRef]
- Friedland, K.D.; Mouw, C.B.; Asch, R.G.; Ferreira, A.S.A.; Henson, S.; Hyde, K.; Morse, R.E.; Thomas, A.C.; Brady, D.C. Phenology and time series trends of the dominant seasonal phytoplankton bloom across global scales. Glob. Ecol. Biogeogr. 2018, 27, 551–569. [Google Scholar] [CrossRef]
- Godin, G. The Analysis of Tides; University of Toronto Press: Toronto, ON, Canada, 1972. [Google Scholar]
- Pawlowicz, R.; Beardsley, B.; Lentz, S. Classical tidal harmonic analysis including error estimates in MATLAB using T_TIDE. Comput. Geosci. 2002, 28, 929–937. [Google Scholar] [CrossRef]
- Jackson, J.; Thomson, R.E.; Brown, L.N.; Willis, P.G.; Borstad, G.A. Satellite chlorophyll off the British Columbia Coast, 1997–2010. J. Geophys. Res. Oceans 2015, 120, 4709–4728. [Google Scholar] [CrossRef]
- Gregg, W.W.; Conkright, M.E. Decadal changes in global ocean chlorophyll. Geophys. Res. Lett. 2002, 29, 20–21. [Google Scholar] [CrossRef] [Green Version]
- Gregg, W.W.; Casey, N.W. Global and regional evaluation of the SeaWiFS chlorophyll data set. Remote Sens. Environ. 2004, 93, 463–479. [Google Scholar] [CrossRef]
- Westberry, T.K.; Schultz, P.; Behrenfeld, M.J.; Dunne, J.P.; Hiscock, M.R.; Maritorena, S.; Sarmiento, J.L.; Siegel, D.A. Annual cycles of phytoplankton biomass in the subarctic Atlantic and Pacific Ocean. Glob. Biogeochem. Cycles 2016, 30, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Jena, B.; Swain, D.; Avinash, K. Investigation of the biophysical processes over the oligotrophic waters of South Indian Ocean subtropical gyre, triggered by cyclone Edzani. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 49–56. [Google Scholar] [CrossRef]
- Zhao, Q.; Costello, M.J. Summer and winter ecosystems of the world ocean photic zone. Ecol. Res. 2019, 34, 457–471. [Google Scholar] [CrossRef]
- Xing, X.-G.; Zhao, D.-Z.; Liu, Y.-G.; Yang, J.-H.; Xiu, P.; Wang, L. An overview of remote sensing of chlorophyll fluorescence. Ocean Sci. J. 2007, 42, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-E.; Lim, J.-H.; Son, S.; Youn, S.-H.; Oh, H.-J.; Hwang, J.-D.; Kwon, J.-I.; Kim, S.-S.; Kim, I.-N. Assessment of Satellite-Based Chlorophyll-a Algorithms in Eutrophic Korean Coastal Waters: Jinhae Bay Case Study. Front. Mar. Sci. 2019, 6, 359. [Google Scholar] [CrossRef]
- Ayers, J.M.; Lozier, M.S. Physical controls on the seasonal migration of the North Pacific transition zone chlorophyll front. J. Geophys. Res. Space Phys. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Racault, M.-F.; Le Quéré, C.; Buitenhuis, E.T.; Sathyendranath, S.; Platt, T. Phytoplankton phenology in the global ocean. Ecol. Indic. 2012, 14, 152–163. [Google Scholar] [CrossRef]
- Yamada, K.; Ishizaka, J.; Yoo, S.; Kim, H.-C.; Chiba, S. Seasonal and interannual variability of sea surface chlorophyll a concentration in the Japan/East Sea (JES). Prog. Oceanogr. 2004, 61, 193–211. [Google Scholar] [CrossRef]
- Li, Y.; Ji, R.; Jenouvrier, S.; Jin, M.; Stroeve, J. Synchronicity between ice retreat and phytoplankton bloom in circum-Antarctic polynyas. Geophys. Res. Lett. 2016, 43, 2086–2093. [Google Scholar] [CrossRef] [Green Version]
- Land, P.E.; Shutler, J.; Platt, T.; Racault, M.-F. A novel method to retrieve oceanic phytoplankton phenology from satellite data in the presence of data gaps. Ecol. Indic. 2014, 37, 67–80. [Google Scholar] [CrossRef]
- Xie, S.-P.; Xie, Q.; Wang, D.; Liu, W.T. Summer upwelling in the South China Sea and its role in regional climate variations. J. Geophys. Res. Space Phys. 2003, 108, 3261. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.P.; Muraleedharan, P.M.; Prasad, T.G.; Gauns, M.; Ramaiah, N.; De Souza, S.N.; Madhupratap, M. Why is the Bay of Bengal less productive during summer monsoon compared to the Arabian Sea? Geophys. Res. Lett. 2002, 29, 88. [Google Scholar]













| Satellite | Date | Number of Days |
|---|---|---|
| SeaWiFS | 4 September 1997–11 December 2010 | 4488 |
| MODIST | 24 February 2000–3 June 2019 | 6953 |
| MERIS | 9 April 2002–8 April 2012 | 3502 |
| MODISA | 4 July 2002–9 July 2019 | 6465 |
| VIIRS | 2 January 2012–8 June 2019 | 2681 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Z.; Mao, Z.; Jamet, C.; Linderman, M.; Wang, Y.; Chen, X. Seasonal Cycles of Phytoplankton Expressed by Sine Equations Using the Daily Climatology from Satellite-Retrieved Chlorophyll-a Concentration (1997–2019) Over Global Ocean. Remote Sens. 2020, 12, 2662. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12162662
Mao Z, Mao Z, Jamet C, Linderman M, Wang Y, Chen X. Seasonal Cycles of Phytoplankton Expressed by Sine Equations Using the Daily Climatology from Satellite-Retrieved Chlorophyll-a Concentration (1997–2019) Over Global Ocean. Remote Sensing. 2020; 12(16):2662. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12162662
Chicago/Turabian StyleMao, Zexi, Zhihua Mao, Cédric Jamet, Marc Linderman, Yuntao Wang, and Xiaoyan Chen. 2020. "Seasonal Cycles of Phytoplankton Expressed by Sine Equations Using the Daily Climatology from Satellite-Retrieved Chlorophyll-a Concentration (1997–2019) Over Global Ocean" Remote Sensing 12, no. 16: 2662. https://0-doi-org.brum.beds.ac.uk/10.3390/rs12162662