Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview
Abstract
:1. Introduction
2. Thermal Treatments and Temperature Monitoring
2.1. Thermal Treatment Modalities: Essential Physics and Applications
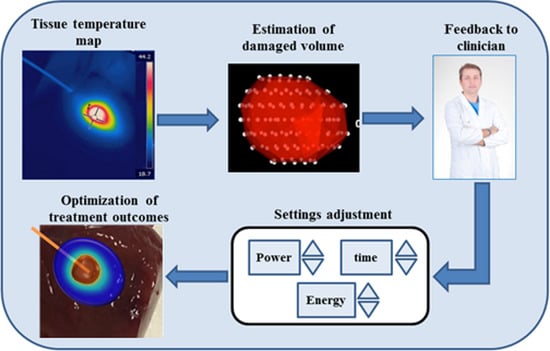
2.2. Temperature Monitoring during Thermal Treatments: Importance and Requirements
3. Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: Working Principles and Metrological Properties
3.1. Fluorescence-Based Sensors
3.2. Fiber Bragg Grating (FBG)
3.3. Chirped FBG
3.4. Rayleigh Scattering Distributed Sensing
4. Application of FOSs in Temperature Monitoring during Thermal Treatment
4.1. Applications of FOSs during Laser Ablation
4.2. Applications of FOSs during Microwave Ablation
4.3. Applications of FOSs during Radiofrequency Ablation
4.4. Temperature Monitoring during HIFU Ablation by FOSs
4.5. Temperature Monitoring during Cryoablation by FOSs
5. Discussion
6. Conclusions
Conflicts of Interest
References
- Ahmed, M.; Brace, C.L.; Lee, F.T.; Goldberg, S.N. Principles of and advances in percutaneous ablation. Radiology 2011, 258, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.N.; Grassi, C.J.; Cardella, J.F.; Charboneau, J.W.; Dodd, G.D.; Dupuy, D.E.; Gervais, D.A.; Gillams, A.R.; Kane, R.A.; Lee, F.T., Jr.; et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria 1. Radiology 2005, 235, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Lepetit-Coiffé, M.; Laumonier, H.; Seror, O.; Quesson, B.; Sesay, M.-B.; Moonen, C.T.W.; Grenier, N.; Trillaud, H. Real-time monitoring of radiofrequency ablation of liver tumors using thermal-dose calculation by MR temperature imaging: Initial results in nine patients, including follow-up. Eur. Radiol. 2010, 20, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Paulides, M.M.; Stauffer, P.R.; Neufeld, E.; Maccarini, P.F.; Kyriakou, A.; Canters, R.A.M.; Diederich, C.J.; Bakker, J.F.; Van Rhoon, G.C. Simulation techniques in hyperthermia treatment planning. Int. J. Hyperth. 2013, 29, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.; Staruch, R.M.; Chopra, R. Thermometry and ablation monitoring with ultrasound. Int. J. Hyperth. 2015, 31, 163–181. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Silvestri, S. Techniques for temperature monitoring during laser-induced thermotherapy: An overview. Int. J. Hyperth. 2013, 29, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Fani, F.; Saccomandi, P.; Schena, E.; Silvestri, S. CT-based thermometry: An overview. Int. J. Hyperth. 2014, 30, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Azhari, H. Feasibility study of ultrasonic computed tomography-guided high-intensity focused ultrasound. Ultrasound Med. Biol. 2012, 38, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Rieke, V.; Pauly, K.B. MR thermometry. J. Magn. Res. Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Arnal, B.; Pernot, M.; Tanter, M. Monitoring of thermal therapy based on shear modulus changes: I. shear wave thermometry. IEEE Trans Ultrason. Ferroelectr. Freq. Control 2011, 58, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Viallon, M.; Terraz, S.; Roland, J. Observation and correction of transient cavitation-induced PRFS thermometry artifacts during radiofrequency ablation, using simultaneous ultrasound/MR. Med. Phys. 2010, 37, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, M.; Daniel, B. MRI-guided radiofrequency ablation of breast cancer: Preliminary clinical experience. J. Magn. Reson. Imaging 2008, 27, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.; Diakite, M.; Payne, A.; Parker, D.L. In vivo evaluation of multi-echo hybrid PRF/T1 approach for temperature monitoring during breast MR-guided focused ultrasound surgery treatments. Magn. Reson. Med. 2014, 72, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Goldberg, S.N.; Sosna, J.; Azhari, H. Temperature-density hysteresis in X-ray CT during HIFU thermal ablation: Heating and cooling phantom study. Int. J. Hyperth. 2013, 30, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Weiss, N.; Sosna, J.; Goldberg, S.N.; Azhari, H. Non-invasive temperature monitoring and hyperthermic injury onset detection using X-ray CT during HIFU thermal treatment in ex vivo fatty tissue. Int. J. Hyperth. 2014, 30, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, G.D.; Klaessens, J.H.G.M.; Greuter, M.J.W.; Schmidt, B.; Flohr, T.; Van Hillegersberg, R.; Oudkerk, M. Feasibility of computed tomography based thermometry during interstitial laser heating in bovine liver. Eur. Radiol. 2011, 21, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- AngioDynamics. Available online: http://www.angiodynamics.com/products/starburst-semiflex (accessed on 20 November 2015).
- Banerjee, R.; Dasgupta, S. Characterization methods of high-intensity focused ultrasound-induced thermal field. Adv. Heat Transf. 2010, 42, 137–177. [Google Scholar]
- Manns, F.; Milne, P.J.; Gonzalez-Cirre, X.; Denham, D.B.; Parel, J.M.; Robinson, D.S. In situ temperature measurements with thermocouple probes during laser interstitial thermotherapy (LITT): Quantification and correction of a measurement artifact. Lasers Surg. Med. 1998, 23, 94–103. [Google Scholar] [CrossRef]
- Rivens, I.; Shaw, A.; Civale, J.; Morris, H. Treatment monitoring and thermometry for therapeutic focused ultrasound. Int. J. Hyperth. 2007, 23, 121–139. [Google Scholar] [CrossRef]
- Taffoni, F.; Formica, D.; Saccomandi, P.; Di Pino, G.; Schena, E. Optical fiber-based MR-compatible sensors for medical applications: An overview. Sensors 2013, 13, 14105–14120. [Google Scholar] [CrossRef] [PubMed]
- Udd, E.; Spillman, W.B., Jr. Fiber Optic Sensors: An Introduction for Engineers and Scientists; John Wiley Sons: New York, NY, USA, 2011. [Google Scholar]
- Stafford, R.J.; Fuentes, D.; Elliott, A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Valcavi, R.; Riganti, F.; Bertani, A.; Formisano, D.; Pacella, C.M. Percutaneous Laser Ablation of Cold Benign Thyroid Nodules: A 3-Year Follow-Up Study in 122 Patients. Thyroid 2010, 20, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Gilling, P.J.; Cass, C.B.; Cresswell, M.D.; Fraundorfer, M.R. Holmium laser resection of the prostate: Preliminary results of a new method for the treatment of benign prostatic hyperplasia. Urology 1996, 47, 48–51. [Google Scholar] [CrossRef]
- Di Matteo, F.M.; Picconi, F.; Martino, M.; Pandolfi, M.; Pacella, C.M.; Schena, E.; Costamagna, G. 350 Endoscopic ultrasound-guided Nd:YAG laser ablation of recurrent pancreatic neuroendocrine tumor: A 351 promising revolution? Endoscopy 2014, 26, E380–E381. [Google Scholar]
- Ierardi, A.; Mangano, A.; Floridi, C.; Dionigi, G. A new system of microwave ablation at 2450 MHz: Preliminary experience. Updates Surg. 2015, 67, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Dupuy, D.; Mayo-Smith, W. Microwave ablation: Principles and applications 1. Radiographics 2005, 25. [Google Scholar] [CrossRef] [PubMed]
- Carrafiello, G.; Lagana, D.; Mangini, M.; Fontana, F.; Dionigi, G.; Boni, L.; Rovera, F.; Cuffari, S.; Fugazzola, C. Microwave tumors ablation: Principles, clinical applications and review of preliminary experiences. Int. J. Surg. 2008, 6, S65–S69. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.N. Radiofrequency tumor ablation: Principles and techniques. Eur. J. Ultrasound 2001, 13, 129–147. [Google Scholar] [CrossRef]
- Pereira, P.L.; Trübenbach, J.; Schenk, M.; Subke, J.; Kroeber, S.; Schaefer, I.; Remy, C.T.; Schmidt, D.; Brieger, J.; Claussen, C.D. Radiofrequency ablation: In vivo comparison of four commercially available devices in pig livers. Radiology 2004, 232, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-intensity focused ultrasound: Current potential and oncologic applications. Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Esnault, O.; Franc, B.; Ménégaux, F.; Rouxel, A.; De Kerviler, E.; Bourrier, P.; Leenhardt, L. High-intensity focused ultrasound ablation of thyroid nodules: First human feasibility study. Thyroid 2011, 21, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Bouley, D.M.; Daniel, B.; Butts Pauly, K.; Liu, E.; Kinsey, A.; Nau, W.; Diederich, C.J.; Sommer, G. Correlation of Contrast-enhanced MR Images with the Histopathology of Minimally Invasive Thermal and Cryoablation Cancer Treatments in Normal Dog Prostates. Available online: http://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC4112763/ (accessed on 20 November 2015).
- Tatli, S.; Acar, M.; Tuncali, K.; Morrison, P.R.; Silverman, S. Percutaneous cryoablation techniques and clinical applications. Diagn. Interv. Radiol. 2010, 16, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Frich, L. Non-invasive thermometry for monitoring hepatic radiofrequency ablation. Minim. Invasive Ther. Allied Technol. 2006, 15, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, N.; Chen, Z. Dynamic temperature monitoring and control with fully distributed fiber Bragg grating sensor. In Proceedings of the Photonics Asia 2010 Internationsl Socisty Optics Photonics, Beijing, China, 18–21 October 2010.
- Abtin, F.G.; Eradat, J.; Gutierrez, A.J.; Lee, C.; Fishbein, M.C.; Suh, R.D. Radiofrequency ablation of lung tumors: Imaging features of the postablation zone. Radiographics 2012, 32, 947–969. [Google Scholar] [CrossRef] [PubMed]
- LumaSense Technologies. Available online: http://www.lumasenseinc.com/ (accessed on 20 November 2015).
- Askins, C.G.; Putman, M.A.; Williams, G.M.; Friebele, E.J. Stepped-wavelength optical-fiber Bragg grating arrays fabricated in line on a draw tower. Opt. Lett. 1994, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Tosi, D.; Macchi, E.G.; Gallati, M.; Braschi, G.; Cigada, A.; Rossi, S.; Leen, G.; Lewis, E. Fiber-optic chirped FBG for distributed thermal monitoring of ex vivo radiofrequency ablation of liver. Biomed. Opt. Express 2014, 5, 1799–1811. [Google Scholar] [CrossRef] [PubMed]
- Macchi, E.G.; Tosi, D.; Braschi, G.; Gallati, M.; Cigada, A.; Busca, G.; Lewis, E. Optical fiber sensors-based temperature distribution measurement in ex vivo radiofrequency ablation with submillimeter resolution. J. Biomed. Opt. 2014, 19, 117004. [Google Scholar] [CrossRef] [PubMed]
- Grattan, K.T.V.; Zhang, Z.Y. Fiber Optic Fluorescence Thermometry; Springer Science & Business Media: Berlin, Germany, 1995. [Google Scholar]
- Zhang, Z.; Grattan, K.; Palmer, A. Fiber-optic high-temperature sensor based on the fluorescence lifetime of alexandrite. Rev. Sci. Instrum. 1992, 63, 3869. [Google Scholar] [CrossRef]
- Berthou, H.; Jörgensen, C.K. Optical-fiber temperature sensor based on upconversion-excited fluorescence. Opt. Lett. 1990, 15, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Katsuki, M.; Mizutani, Y. Measurement of flame temperature by optical fiber thermometer. Exp. Fluids 1988, 6, 514–520. [Google Scholar] [CrossRef]
- Yu, Y.B.; Chow, W.K. Review on an Advanced High-Temperature Measurement Technology: The Optical Fiber Thermometry. J. Thermodyn. 2009, 2009, 1–11. [Google Scholar] [CrossRef]
- Wickersheim, K.; Sun, M. Fiberoptic thermometry and its applications. J. Microw. Power 1987, 22, 85–94. [Google Scholar]
- Vaguine, V. Multiple sensor optical thermometry system for application in clinical hyperthermia. IEEE Trans. Bio-Med. Eng. 1984, 1, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, T. Fiber grating spectra. J. Light. Technol. 1997, 15, 1277–1294. [Google Scholar] [CrossRef]
- Tosi, D.; Macchi, E.; Cigada, A. Fiber-Optic Temperature and Pressure Sensors Applied to Radiofrequency Thermal Ablation in Liver Phantom: Methodology and Experimental Measurements. J. Sens. 2015, 2015, 909012. [Google Scholar] [CrossRef]
- Tosi, D.; Macchi, E.; Braschi, G.; Gallati, M. Monitoring of radiofrequency thermal ablation in liver tissue through fibre Bragg grating sensors array. Electron. Lett. 2014, 50, 981–983. [Google Scholar] [CrossRef]
- Johnson, D. Novel Optical Fibers-Draw-tower process creates high-quality FBG arrays. Laser Focus World 2012, 48, 53. [Google Scholar]
- FBGS. Available online: http://www.fbgs.com/ (accessed on 20 November 2015).
- Geernaert, T.; Kalli, K.; Koutsides, C. Point-by-point fiber Bragg grating inscription in free-standing step-index and photonic crystal fibers using near-IR femtosecond laser. Opt. Lett. 2010, 35, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Gifford, D.; Soller, B.; Wolfe, M.; Froggatt, M. Distributed fiber-optic temperature sensing using Rayleigh backscatter. ECOC Proc. 2005, 3, 511–512. [Google Scholar]
- Froggatt, M.E.; Gifford, D.K.; Kreger, S.; Wolfe, M.; Soller, B.J. Characterization of polarization-maintaining fiber using high-sensitivity optical-frequency-domain reflectometry. J. Light. Technol. 2006, 24, 4149–4154. [Google Scholar] [CrossRef]
- Luna. Available online: http://lunainc.com/obr4600 (accessed on 20 November 2015).
- Rao, Y.J.; Webb, D.J.; Jackson, D.A.; Zhang, L.; Bennion, I. In-fiber bragg-grating temperature sensor system for medical applications. J. Light. Technol. 1997, 15, 779–784. [Google Scholar]
- Rao, Y.J.; Webb, D.J.; Jackson, D.A.; Zhang, L.; Bennion, I. Optical in-fiber bragg grating sensor systems for medical applications. J. Biomed. Opt. 1998, 3, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Webb, D.J.; Hathaway, M.W.; Jackson, D.A.; Jones, S.; Zhang, L.; Bennion, I. First in vivo trials of a fiber Bragg grating based temperature profiling system. J. Biomed. Opt. 2000, 5, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.R.H.; Vitkin, I.A.; Sherar, M.D.; Whelan, W.M. Characterization of measurement artefacts in fluoroptic temperature sensors: Implications for laser thermal therapy at 810 nm. Lasers Surg. Med. 2005, 36, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.D.; Gertner, M.R.; Sherar, M.D. Temperature measurement artefacts of thermocouples and fluoroptic probes during laser irradiation at 810 nm. Phys. Med. Biol. 2001, 46, N149–N157. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chen, S.; Zhu, H.; Liu, S.; Chen, Z.; Pang, F.; Wang, T. In vivo experiments of laser thermotherapy on liver tissue with FBG temperature distribution sensor. In Proceedings of the Photonic Microdevices/Microstructures for Sensing IV, Baltimore, MD, USA, 26–27 April 2012.
- Saccomandi, P.; Schena, E.; Caponero, M.A.; Di Matteo, F.M.; Martino, M.; Pandolfi, M.; Silvestri, S. Theoretical analysis and experimental evaluation of laser-induced interstitial thermotherapy in ex vivo porcine pancreas. IEEE Trans. Biomed. Eng. 2012, 59, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Giurazza, F.; Del Vescovo, R.; Caponero, M.A.; Mortato, L.; Panzera, F.; Cazzato, R.L.; Grasso, F.R.; Di Matteo, F.M.; et al. Temperature monitoring and lesion volume estimation during double-applicator laser-induced thermotherapy in ex vivo swine pancreas: A preliminary study. Lasers Med. Sci. 2014, 29, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Lupi, G.; Schena, E.; Polimadei, A.; Caponero, M.A.; Panzera, F.; Martino, M.; Di Matteo, F.M.; Sciuto, S.; Silvestri, S. Influence of FBG sensors length on temperature measures in laser-irradiated pancreas: Theoretical and experimental evaluation. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2013, 2013, 3737–3740. [Google Scholar]
- Polito, D.; Caponero, M.A.; Polimadei, A.; Saccomandi, P.; Massaroni, C.; Silvestri, S.; Schena, E. A needle-like probe for temperature monitoring during laser ablation based on FBG: Manufacturing and characterization. J. Med. Device 2015, 9. [Google Scholar] [CrossRef]
- Cappelli, S.; Saccomandi, S.; Massaroni, C.; Polimadei, A.; Silvestri, S.; Caponero, M.A.; Frauenfelder, G.; Schena, E. Magnetic Resonance-compatible needle-like probe based on Bragg grating technology for measuring temperature during Laser Ablation. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 1287–1290.
- Allegretti, G.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Frauenfelder, G.; Di Matteo, F.M.; Beomonte Zobel, B.; Silvestri, S.; Schena, E. Magnetic resonance-based thermometry during laser ablation on ex vivo swine pancreas and liver. Med. Eng. Phys. 2015, 37, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Mortato, L.; Di Matteo, F.M.; Panzera, F.; Del Vescovo, R.; Beomonte Zobel, B.; Silvestri, S. Experimental assessment of CT-based thermometry during laser ablation of porcine pancreas. Phys. Med. Biol. 2013, 58, 5705–5716. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, W.; Yu, H.; Gassino, R.; Braglia, A.; Olivero, M.; Perrone, G.; Vallan, A. All-fiber probe for laser-induced thermotherapy with integrated temperature measurement capabilities. In Proceedings of the Optical Fibers and Sensors for Medical Diagnostics and Treatment Applications XV Israel Gannot, San Francisco, CA, USA, 7–8 February 2015.
- Demura, K.; Morikawa, S.; Murakami, K.; Sato, K.; Shiomi, H.; Naka, S.; Kurumi, Y.; Inubushi, T.; Tani, T. An Easy-to-Use Microwave Hyperthermia System Combined with Spatially Resolved MR Temperature Maps: Phantom and Animal Studies. J. Surg. Res. 2006, 135, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Converse, M.C.; Mahvi, D.M.; Webster, J.G. Measurement and analysis of tissue temperature during microwave liver ablation. IEEE Trans. Biomed. Eng. 2007, 54, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.; Hernández, A. Coaxial Slot Antenna Design for Microwave Hyperthermia using Finite-Difference Time-Domain and Finite Element Method. Open Nanomed. J. 2011, 3, 2–9. [Google Scholar] [CrossRef]
- Chen, J.C.; Moriarty, J.A.; Derbyshire, J.A.; Peters, R.D.; Trachtenberg, J.; Bell, S.D.; Doyle, J.; Arrelano, R.; Wright, G.A.; Henkelman, R.M.; et al. Prostate cancer: MR imaging and thermometry during microwave thermal ablation-initial experience. Radiology 2000, 214, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.F.; Hui, K.; Astrahan, M. Polymer coated fiber Bragg grating thermometry for microwave hyperthermia. Med. Phys. 2010, 37, 4615–4619. [Google Scholar] [CrossRef] [PubMed]
- Saccomandi, P.; Schena, E.; Massaroni, C.; Fong, Y.; Grasso, R.F.; Giurazza, F.; Beomonte Zobel, B.; Buy, X.; Palussiere, J.; Cazzato, R.L. Temperature monitoring during microwave ablation in ex vivo porcine livers. Eur. J. Surg. Oncol. 2015, 41, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, C.P.A.; Leija, L.; Fonseca, W.H.; Vera, A. Fiber Optic Temperature Sensor for Use in Experimental Microwave Hyperthermia. In Proceedings of the IEEE Sensors, Orlando, FL, USA, 12–14 June 2002.
- Ji, Z.; Brace, C.L. Expanded modeling of temperature-dependent dielectric properties for microwave thermal ablation. Phys. Med. Biol. 2011, 56, 5249–5264. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Wittkampf, F.H.; Yamanashi, W.S.; Pitha, J.V.; Imai, S.; Campbell, B.; Arruda, M.; Lazzara, R.; Jackman, W.M. Inverse relationship between electrode size and lesion size during radiofrequency ablation with active electrode cooling. Circulation 1998, 98, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, S.A.; Liu, Z.; Lobo, S.M.; Ahmed, M.; Hines-Peralta, A.U.; Lenkinski, R.E.; Goldberg, S.N. Radiofrequency ablation: Importance of background tissue electrical conductivity-an agar phantom and computer modeling study. Radiology 2005, 236, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ahmed, M.; Weinstein, Y. Characterization of the RF ablation-induced “oven effect”: The importance of background tissue thermal conductivity on tissue heating. Int. J. Hyperth. 2006, 22, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Lobik, L.; Leveillee, R.; Hoey, M. Geometry and temperature distribution during radiofrequency tissue ablation: An experimental ex vivo model. J. Endourol. 2005, 19, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Nakagawa, H.; Shah, D.C.; Lambert, H.; Leo, G.; Aeby, N.; Ikeda, A.; Pitha, J.V.; Sharma, T.; Lazzara, R.; et al. Novel contact force sensor incorporated in irrigated radiofrequency ablation catheter predicts lesion size and incidence of steam pop and thrombus. Circ. Arrhythm. Electrophysiol. 2008, 1, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Bohris, C.; Schreiber, W. Quantitative MR temperature monitoring of high-intensity focused ultrasound therapy. Magn. Reson. Imaging 1999, 17, 603–610. [Google Scholar] [CrossRef]
- Jenne, J.; Bahner, M.; Spoo, J. CT on-line monitoring of HIFU therapy. IEEE Ultrason. Symp. 1997, 2, 1377–1380. [Google Scholar]
- Wong, S.H.; Kupnik, M.; Butts-Pauly, K.; Khuri-Yakub, B.T. P18-10 Advantages of Capacitive Micromachined Ultrasonics Transducers (CMUTs) for High Intensity Focused Ultrasound (HIFU). In Proceedings of the 2007 IEEE on Ultrasonics Symposium, New York, NY, USA, 28–31 October 2007.
- Ranjan, A.; Jacobs, G.; Woods, D. Image-guided drug delivery with magnetic resonance guided high intensity focused ultrasound and temperature sensitive liposomes in a rabbit Vx2 tumor model. J. Control Release 2012, 158, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Petrusca, L.; Salomir, R. Spatio-temporal quantitative thermography of pre-focal interactions between high intensity focused ultrasound and the rib cage. Int. J. Hyperth. 2015, 31, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.; Hurrell, A.; Shaw, A.; Zhang, E.; Beard, P. A Fabry-Perot fiber-optic ultrasonic hydrophone for the simultaneous measurement of temperature and acoustic pressure. J. Acoust. Soc. Am. 2009, 125, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.; Wilkens, V. Characterization of a fiber-optic displacement sensor for measurements in high-intensity focused ultrasound fields. J. Acoust. Soc. Am. 2011, 129, 3676–3681. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, S.; Jia, P. In-line silica capillary tube all-silica fiber-optic Fabry-Perot interferometric sensor for detecting high intensity focused ultrasound fields. Opt. Lett. 2012, 37, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jia, P.; Ma, Z. Tip-sensitive fibre-optic Bragg grating ultrasonic hydrophone for measuring high-intensity focused ultrasound fields. Electron. Lett. 2014, 50, 649–650. [Google Scholar] [CrossRef]
- Favazza, C.P.; Gorny, K.R.; King, D.M.; Rossman, P.J.; Felmlee, J.P.; Woodrum, D.A.; Mynderse, L.A. An investigation of the effects from a urethral warming system on temperature distributions during cryoablation treatment of the prostate: A phantom study. Cryobiology 2014, 69, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Samset, E.; Mala, T.; Edwin, B.; Gladhaug, I.; Søreide, O.; Fosse, E. Validation of estimated 3D temperature maps during hepatic cryo surgery. Magn. Reson. Imaging 2001, 19, 715–721. [Google Scholar] [CrossRef]
- Samset, E.; Mala, T.; Ellingsen, R.; Gladhaug, I.; Søreide, O.; Fosse, E. Temperature measurement in soft tissue using a distributed fibre Bragg-grating sensor system. Minim. Invasive Ther. Allied Technol. 2001, 10, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Gowardhan, B.; Greene, D. Cryotherapy for the prostate: An in vitro and clinical study of two new developments; advanced cryoneedles and a temperature monitoring system. BJU Int. 2007, 100, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rothgang, E.; Gilson, W.D.; Strehl, W.; Pan, L.; Roland, J.; Lorenz, C.H.; Hornegger, J. Interventional MR-imaging for thermal ablation therapy. In Proceedings of the IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Chicago, IL, USA, 30 March–2 April 2011; pp. 1864–1868.
- Tosi, D.; Saccomandi, P.; Schena, E.; Duraibabu, D.B.; Poeggel, S.; Leen, G.; Lewis, E. Intra-Tissue Pressure Measurement in ex vivo Liver Undergoing Laser Ablation with Fiber-Optic Fabry-Perot Probe. Sensors 2016, 16, 544. [Google Scholar] [CrossRef] [PubMed]




| First Author, Year, Ref | Kind of FOS | Thermal Treatment | Model (in vivo, ex vivo) | Kind of Sensor, Number, Size, Embedding | Features (Accuracy, Errors, Measurement Range, Constant Time, Frequency Response, Sensitivity) |
|---|---|---|---|---|---|
| Davidson et al., 2005 [65] | Fluoroptic sensors | LA | air, water | 4 sensors | |
| Reid et al., 2001 [66] | Fluoroptic sensors | LA | air, water, agar–albumen phantom | ||
| Yang et al., 2007 [77] | Fluoroptic sensors | MWA | ex vivo bovine liver | 4 sensors inserted through biopsy needles | 8–120 °C |
| Rubio et al., 2011 [78] | Fluoroptic sensors | MWA | ex vivo swine muscle | 4 sensors | 19–60 °C |
| Chen et al., 2000 [79] | Fluoroptic sensors | MWA | in vivo patients (prostate cancer) | 2 sensors | measurement range: 37–70 °C |
| Nakagawa et al., 1998 [84], 2008 [88] | Fluoroptic sensors | RFA | In vivo canine model | 4 sensors | |
| Solazzo et al., 2005 [85] | Fluoroptic sensors | RFA | Agar phantom | ||
| Lobik et al., 2005 [87] | Fluoroptic sensors | RFA | Egg phantom | ||
| van den Bosch et al., 2008 [12] | Fluoroptic sensors | RFA | 3 women affected with breast cancer | 4 sensors | |
| Viallon et al., 2010 [11] | Fluoroptic sensors | RFA | ex vivo tissue | ||
| Bohris et al., 1995 [89] | Fluoroptic sensors | HIFU | ex vivo porcine muscle and fat | ||
| Jenne et al., 1997 [90] | Fluoroptic sensors | HIFU | ex vivo porcine muscle | ||
| Wong et al., 2007 [91] | Fluoroptic sensors | HIFU | oil phantom | ||
| Ranjan et al., 2012 [92] | Fluoroptic sensors | HIFU | in vivo rabbit Vx2 tumor models | Neoptix T1 probe, Fluoroptic sensors | |
| Petrusca et al., 2015 [93] | Fluoroptic sensors | HIFU | ex vivo turkey tissue | Fluoroptic sensors | |
| Bouley et al., 2007 [36] | Fluoroptic sensors | Cryo | in vivo dog prostate | 4 sensors | |
| Favazza et al., 2014 [98] | Fluoroptic sensors | Cryo | prostate mimicking phantom | 4 sensors | |
| Saccomandi et al., 2012–2014 [68,69,70] | FBG | LA | ex vivo porcine pancreas | 6 FBGs, 1 mm and 10 mm of length, non-encapsulated | |
| Polito et al., 2015 [71] | FBG | LA | ex vivo porcine liver | FBG 10 mm of length, encapsulated in metallic needle | measurement range: 20–80 °C sensitivity: from 0.01 nm∙°C−1 to 0.027 nm∙°C−1 time constant: 100 ms |
| Cappelli et al., 2015 [72] | FBG | LA | ex vivo porcine liver | 3 FBGs 1mm of length, encapsulated in MRI compatible needle | measurement range: 20–80 °C sensitivity: 0.01 nm∙°C−1 time constant: 100 ms |
| Schena et al., 2013–2015 [73,74] | FBG | LA | ex vivo porcine liver and pancreas | 4 FBGs 1mm of length, non-encapsulated | |
| Liu et al., 2015 [75] | FBG | LA | phantom | laser fiber integrating 2 FBGs | time constant: 100 ms |
| Saxena et al., 2010 [80] | FBG | MWA | muscle equivalent phantom | 10 FBGs at distance of 5mm on fiber with 0.125 mm of diameter; coated with polymer (diameter of 0.5 mm) | measurement range: 20 °C–60 °C sensitivity: 23 ± 7 pm·°C−1 Accuracy: 0.25 °C stability over 10 h: 0.5 °C time constant: 2 s |
| Saccomandi et al., 2015 [81] | FBG | MWA | ex vivo porcine liver | FBG sensors (1cm of length) | |
| Tosi et al., 2014 [54] | FBG | RFA | ex vivo porcine liver | 5 FBGs, 5 mm of length mounted on RF needle | FBG spectral spacing: 1.8 nm; Thermo-optic FBG coefficient: 11.66 pm·°C−1. |
| Tosi et al., 2014 [44] | FBG | RFA | ex vivo porcine liver | linearly chirped FBG 15 mm of length | Measurement range: 22–95 °C; Linear chirp parameter: 2.22 nm/mm; Thermo-optic coefficient: 10.2 pm·°C−1. |
| Tosi et al., 2015 [55] | FBG | RFA | ex vivo porcine liver | FBG array, linearly chirped FBG, Fabry–Pérot interferometer for pressure and temperature | see previous studies |
| Samset et al., 2001 [99,100] | FBG | Cryo | in vivo porcine liver | 10 FBGs; 58.5 mm of length, outer diameter of 1.4mm | measurement range: −185–100 °C |
| Pennisi et al., 2002 [82] | change of refractive index | MWA | phantom | 1 sensor based on change of refractive index of medium surrounding cladding; 20 mm of length | measurement range: 18–50 °C |
| Ji et al., 2011 [83] | band gap GaAs sensors | MWA | ex vivo bovine liver | 4 band gap GaAs sensors; 0.4 mm GaAs sensitive area; probe material PTFE | measurement range: 20–130 °C Accuracy: 1 °C time constant: 250 ms |
| Macchi et al., 2014 [45] | DTS system | RFA | ex vivo porcine liver | DTS system based on swept laser interferometry | Spatial resolution: 0.2 mm; accuracy 0.5 °C; active region: 8 fiber spans, 3.6 cm each. |
| Morris et al., 2009 [95] | Fabry–Pérot interferometer | HIFU | oil-gelatin phantom | Fabry–Pérot interferometer | Measurement range: 25–80 °C (linear up to 70 °C); Resolution: 0.34 °C; rate of measurable temperature change: 67 °C·s−1. |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schena, E.; Tosi, D.; Saccomandi, P.; Lewis, E.; Kim, T. Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview. Sensors 2016, 16, 1144. https://0-doi-org.brum.beds.ac.uk/10.3390/s16071144
Schena E, Tosi D, Saccomandi P, Lewis E, Kim T. Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview. Sensors. 2016; 16(7):1144. https://0-doi-org.brum.beds.ac.uk/10.3390/s16071144
Chicago/Turabian StyleSchena, Emiliano, Daniele Tosi, Paola Saccomandi, Elfed Lewis, and Taesung Kim. 2016. "Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview" Sensors 16, no. 7: 1144. https://0-doi-org.brum.beds.ac.uk/10.3390/s16071144