Electrochemical Sensing of α-Fetoprotein Based on Molecularly Imprinted Polymerized Ionic Liquid Film on a Gold Nanoparticle Modified Electrode Surface
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. (Cys)VIMBF4 Ionic Liquid Synthesis
2.3. Gold Nanoparticles Modified Glassy Carbon Electrode Preparation.
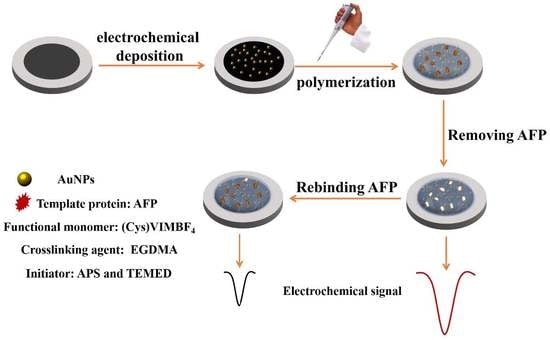
2.4. AFP Molecularly Imprinted Sensor Fabrication
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Characterizations
3.2. Optimizing Experimental Conditions
3.2.1. Effect of the pH Value
3.2.2. Effect of the Incubation Time
3.3. Analytical Characteristics
3.4. Selectivity of the Imprinted Sensor
3.5. Reproducibility and Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wright, L.M.; Kreikemeier, J.T.; Fimmel, C.J. A concise review of serum markers for hepatocellular cancer. Cancer Detect. Prev. 2007, 31, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Li, X.Q.; Wang, H.; Wu, X.M.; Wang, G.L. Tuning surface states to achieve the modulated fluorescence of carbon dots for probing the activity of alkaline phosphatase and immunoassay of alpha-fetoprotein. Sens. Actuators B Chem. 2018, 257, 620–628. [Google Scholar] [CrossRef]
- Niu, Y.L.; Yang, T.; Ma, S.S.; Peng, F.; Yi, M.H.; Wan, M.M.; Mao, C.; Shen, J. Label-free immunosensor based on hyperbranched polyester for specific detection of alpha-fetoprotein. Biosens. Bioelectron. 2017, 92, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Shen, H.Y.; Zhang, G.J.; Jiang, X.Y.; Kang, X.X. Chemiluminescence immunoassay based on microfluidic chips for α-fetoprotein. Clin. Chim. Acta 2014, 431, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Skog, S.; Wang, S.Q.; Ke, Y.; Zhang, Y.H.; Li, K.; He, E.; Li, N. A Chemiluminescent protein microarray method for determining the seroglycoid fucosylation index. Sci. Rep. 2016, 6, 31132. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.L.; Bao, N.; Luo, X.L.; Ding, S.N. CdZnTeS quantum dots based electrochemiluminescent image immunoanalysis. Biosens. Bioelectron. 2018, 117, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Hua, X.X.; Qiao, X.Y.; Xia, F.Q.; Tian, D.; Zhou, C.L. Simple and signal-off electrochemiluminescence immunosensor for alpha fetoprotein based on gold nanoparticle-modified graphite-like carbon nitride nanosheet nanohybrids. RSC Adv. 2016, 6, 21308–21316. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Siangproh, W.; Khongchareonporn, N.; Ngamrojanavanich, N.; Chailapakul, O. Development of an automated wax-printed paper-based lateral flow device for alpha-fetoprotein enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2018, 102, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Wangkam, T.; Boonperm, K.; Khomkrachang, P.; Srikhirin, T.; Praphanphoj, V.; Sutapan, B.; Somboonkaew, A.; Amarit, R. Hepatocellular carcinoma biomarker detection by surface plasmon resonance sensor. Adv. Mater. Res. 2016, 1131, 84–87. [Google Scholar] [CrossRef]
- Yang, S.H.; Zhang, F.F.; Wang, Z.H.; Liang, Q.L. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Qu, Y.; Ye, X.X.; Wu, K.B.; Li, C.Y. Fabrication of an electrochemical immunosensor for alpha-fetoprotein based on a poly-L-lysine-single-walled carbon nanotubes/prussian blue composite film interface. J. Solid State Electrochem. 2016, 20, 2217–2222. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, H.J.; Zhang, Y.Y.; Lv, Y.Q.; Li, H.G.; Liu, S.Q.; Shen, Y.F.; Zhang, Y.J. Metal-free all-carbon nanohybrid for ultrasensitive photoelectrochemical immunosensing of alpha-fetoprotein. ACS Sens. 2018, 3, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, W.; Jiang, Y.D.; Pan, G.C.; Zhou, D.L.; Zhu, J.Y.; Wang, H.; Chen, C.; Li, D.Y.; Song, H.W. A novel upconversion luminescence derived photoelectrochemical immunoassay: Ultrasensitive detection to alpha-fetoprotein. Nanoscale 2017, 9, 16357–16364. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.X.; Tang, Z.G.; Guo, X.X.; Ma, X.M.; Liu, C.B. Computer-aided design of magnetic dummy molecularly imprinted polymers for solid-phase extraction of ten phthalates from food prior to their determination by GC-MS/MS. Microchim. Acta 2018, 185, 373. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Plotka-Wasylka, J.; Morrison, C.; Wieczorek, P.P.; Namiesnik, J.; Marc, M. Application of molecularly imprinted polymers in analytical chiral separations and analysis. TrAC Trends Anal. Chem. 2018, 102, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Mirata, F.; Resmini, M. Molecularly Imprinted Polymers in Biotechnology. In Molecularly Imprinted Polymers for Catalysis and Synthesis; Springer: Cham, Switzerland, 2015; Volume 150, pp. 107–129. [Google Scholar]
- Zhang, X.; Yang, S.; Jiang, R.; Sun, L.Q.; Pang, S.P.; Luo, A.Q. Fluorescent molecularly imprinted membranes as biosensor for the detection of target protein. Sens. Actuators B Chem. 2018, 254, 1078–1086. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.Y.; Ye, X.X.; Wu, T.S.; Deng, H.P.; Wu, P.; Li, C.Y. Sensing platform for neuron specific enolase based on molecularly imprinted polymerized ionic liquids in between gold nanoarrays. Biosens. Bioelectron. 2018, 99, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Ye, X.X.; Wang, Z.G.; Wu, T.S.; Wang, Y.Y.; Li, C.Y. Molecularly imprinted photo-electrochemical sensor for human epididymis protein 4 based on polymerized ionic liquid hydrogel and gold nanoparticle/ZnCdHgSe quantum dots composite film. Anal. Chem. 2017, 89, 12391–12398. [Google Scholar] [CrossRef]
- Yanez-Sedeno, P.; Campuzano, S.; Pingarron, J.M. Electrochemical sensors based on magnetic molecularly imprinted polymers: A review. Anal. Chim. Acta 2017, 960, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mo, G.C.; He, X.X.; Zhou, C.Q.; Ya, D.M.; Feng, J.S.; Yu, C.H.; Deng, B.Y. A novel ECL sensor based on a boronate affinity molecular imprinting technique and functionalized SiO2@CQDs/AuNPs/MPBA nanocomposites for sensitive determination of alpha-fetoprotein. Biosens. Bioelectron. 2019, 126, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.X.; Zhang, C.X.; Deng, Y.; Yang, G.J.; Li, S.; Tang, C.L.; He, N.Y. A novel alpha-fetoprotein-MIP immunosensor based on AuNPs/PTh modified glass carbon electrode. Chin. Chem. Lett. 2019, 30, 160–162. [Google Scholar] [CrossRef]
- Shen, X.L.; Ma, Y.; Zeng, Q.; Tao, J.; Wang, L.S. Molecularly imprinted electrochemical sensor for advanced diagnosis of alpha-fetoprotein. Anal. Methods 2016, 8, 7361–7368. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Han, M.; Ye, X.X.; Wu, K.B.; Wu, T.S.; Li, C.Y. Voltammetric myoglobin sensor based on a glassy carbon electrode modified with a composite film consisting of carbon nanotubes and a molecularly imprinted polymerized ionic liquid. Microchim. Acta 2017, 184, 195–202. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Han, M.; Liu, G.S.; Hou, X.D.; Huang, Y.N.; Wu, K.B.; Li, C.Y. Molecularly imprinted electrochemical sensing interface based on in-situ-polymerization of amino-functionalized ionic liquid for specific recognition of bovine serum albumin. Biosens. Bioelectron. 2015, 74, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Li, C.Y. Synthesis and characterization of 1-(3-(N-Cysimine) propyl)-3-methylimidazolium bromine ionic liquid. GuangZhou Chem. Ind. 2015, 43, 56–58. [Google Scholar]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, Y.; Wang, X.; Wang, C.; Li, C.; Wang, Z. Electrochemical Sensing of α-Fetoprotein Based on Molecularly Imprinted Polymerized Ionic Liquid Film on a Gold Nanoparticle Modified Electrode Surface. Sensors 2019, 19, 3218. https://0-doi-org.brum.beds.ac.uk/10.3390/s19143218
Wu Y, Wang Y, Wang X, Wang C, Li C, Wang Z. Electrochemical Sensing of α-Fetoprotein Based on Molecularly Imprinted Polymerized Ionic Liquid Film on a Gold Nanoparticle Modified Electrode Surface. Sensors. 2019; 19(14):3218. https://0-doi-org.brum.beds.ac.uk/10.3390/s19143218
Chicago/Turabian StyleWu, Yingying, Yanying Wang, Xing Wang, Chen Wang, Chunya Li, and Zhengguo Wang. 2019. "Electrochemical Sensing of α-Fetoprotein Based on Molecularly Imprinted Polymerized Ionic Liquid Film on a Gold Nanoparticle Modified Electrode Surface" Sensors 19, no. 14: 3218. https://0-doi-org.brum.beds.ac.uk/10.3390/s19143218