Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Synthesis
2.2. Electrode Preparation and Modification
2.3. Instrumentation and Methodology
3. Results and Discussion
3.1. Surface Characterization
3.2. Electrochemical Characterization of the Fabricated Various GO-Based Electrodes
3.3. Electrochemical Behaviors of Naproxen at the Modified Electrodes
3.4. Electrode Fouling
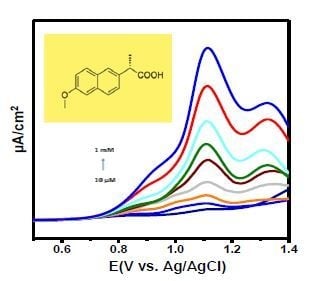
3.5. Electrochemical Sensing of Naproxen
3.6. Interference Studies and Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Angiolillo, D.J.; Weisman, S.M. Clinical pharmacology and cardiovascular safety of naproxen. Am. J. Cardiovasc. Drugs 2017, 17, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Jain, P.S. A concise review on analytical profile of naproxen. IP Int. J. Compr. Adv. Pharmacol. 2019, 4, 70–81. [Google Scholar] [CrossRef]
- Janssens, H.J.E.M.; Janssen, M.; van de Lisdonk, E.H.; van Riel, P.L.C.M.; van Weel, C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: A double-blind, randomised equivalence trial. Lancet 2008, 371, 1854–1860. [Google Scholar] [CrossRef]
- Al-Abri, S.A.; Anderson, I.B.; Pedram, F.; Colby, J.M.; Olson, K.R. Massive naproxen overdose with serial serum levels. J. Med Toxicol. 2015, 11, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of naproxen and its phototransformation products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef]
- Brozinski, J.M.; Lahti, M.; Meierjohann, A.; Oikari, A.; Kronberg, L. The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant. Environ. Sci. Technol. 2013, 47, 342–348. [Google Scholar] [CrossRef]
- Ostojic, J.; Herenda, S.; Besic, Z.; Milos, M.; Galic, B. Advantages of an electrochemical method compared to the spectrophotometric kinetic study of peroxidase inhibition by boroxine derivative. Molecules 2017, 22, 1220. [Google Scholar] [CrossRef] [Green Version]
- Afkhami, A.; Kafrashi, F.; Ahmadi, M.; Madrakian, T. A new chiral electrochemical sensor for the enantioselective recognition of naproxen enantiomers using l-cysteine self-assembled over gold nanoparticles on a gold electrode. RSC Adv. 2015, 5, 58609–58615. [Google Scholar] [CrossRef]
- Baj-Rossi, C.; Rezzonico Jost, T.; Cavallini, A.; Grassi, F.; De Micheli, G.; Carrara, S. Continuous monitoring of Naproxen by a cytochrome P450-based electrochemical sensor. Biosens. Bioelectron. 2014, 53, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Hendawy, H.A.M.; Salem, W.M.; Abd-Elmonem, M.S.; Khaled, E. Nanomaterial-based carbon paste electrodes for voltammetric determination of naproxen in presence of its degradation products. J. Anal. Methods Chem. 2019, 5381031. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Coroş, M.; Pogăcean, F.; Măgeruşan, L.; Socaci, C.; Pruneanu, S. A brief overview on synthesis and applications of graphene and graphene-based nanomaterials. Front. Mater. Sci. 2019, 13, 23–32. [Google Scholar] [CrossRef]
- Rao, S.; Upadhyay, J.; Polychronopoulou, K.; Umer, R.; Das, R. Reduced graphene oxide: Effect of reduction on electrical conductivity. J. Compos. Sci. 2018, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Schniepp, H.C.; Adamson, D.H. Characterization of graphene oxide: Variations in reported approaches. Carbon 2019, 154, 510–521. [Google Scholar] [CrossRef]
- Priya Swetha, P.D.; Manisha, H.; Sudhakaraprasad, K. Graphene and graphene-based materials in biomedical science. Part. Part. Syst. Charact. 2018, 35, 1800105. [Google Scholar] [CrossRef]
- Wang, Z.; Pu, Y.; Wang, D.; Wang, J.-X.; Chen, J.-F. Recent advances on metal-free graphene-based catalysts for the production of industrial chemicals. Front. Chem. Sci. Eng. 2018, 12, 855–866. [Google Scholar] [CrossRef]
- Lee, M.; Yang, S.; Kim, K.-j.; Kim, S.; Lee, H. Comparison of the catalytic oxidation reaction on graphene oxide and reduced graphene oxide. J. Phys. Chem. C 2014, 118, 1142–1147. [Google Scholar] [CrossRef]
- Kong, X.K.; Chen, C.L.; Chen, Q.W. Doped graphene for metal-free catalysis. Chem. Soc. Rev. 2014, 43, 2841–2857. [Google Scholar] [CrossRef]
- Tian, W.C.; Li, W.H.; Yu, W.B.; Liu, X.H. A review on lattice defects in graphene: Types, generation, effects and regulation. Micromachines 2017, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Feicht, P.; Eigler, S. Defects in graphene oxide as structural motifs. ChemNanoMat 2018, 4, 244–252. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537–4554. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.-R.; Govindhan, M.; Chen, A. Sensitive detection of acetaminophen with graphene-based electrochemical sensor. Electrochim. Acta 2015, 162, 198–204. [Google Scholar] [CrossRef]
- Liu, Z.; Puumala, E.; Chen, A. Sensitive electrochemical detection of Hg(II) via a FeOOH modified nanoporous gold microelectrode. Sens. Actuators B Chem. 2019, 287, 517–525. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of graphene-based materials for detection of nitrate and nitrite in water-a review. Sensors 2019, 20, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-based sensors for human health monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Peng, T.; Yuan, M.; Sun, H.; Dai, S.; Wang, L. Preparation of graphite oxide containing different oxygen-containing functional groups and the study of ammonia gas sensitivity. Sensors 2018, 18, 3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.S.; Panesar, D.K.; Sidhureddy, B.; Chen, A.; Wood, P.C. Nano-cement composite with graphene oxide produced from epigenetic graphite deposit. Compos. Part B Eng. 2019, 159, 248–258. [Google Scholar] [CrossRef]
- Thiruppathi, A.R.; Sidhureddy, B.; Keeler, W.; Chen, A. Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 2017, 76, 42–46. [Google Scholar] [CrossRef]
- Guo, H.-L.; Su, P.; Kang, X.; Ning, S.-K. Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A 2013, 1, 2248–2255. [Google Scholar] [CrossRef]
- Iakovlev, V.Y.; Sklyueva, Y.A.; Fedorov, F.S.; Rupasov, D.P.; Kondrashov, V.A.; Grebenko, A.K.; Mikheev, K.G.; Gilmutdinov, F.Z.; Anisimov, A.S.; Mikheev, G.M.; et al. Improvement of optoelectronic properties of single-walled carbon nanotube films by laser treatment. Diam. Relat. Mater. 2018, 88, 144–150. [Google Scholar] [CrossRef]
- Susi, T.; Pichler, T.; Ayala, P. X-ray photoelectron spectroscopy of graphitic carbon nanomaterials doped with heteroatoms. Beilstein J. Nanotechnol. 2015, 6, 177–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, C.; Zhang, Y.; Cummings, P.T.; Jiang, D.E. Enhancing graphene capacitance by nitrogen: Effects of doping configuration and concentration. Phys. Chem. Chem. Phys. 2016, 18, 4668–4674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Hu, N.; Zhang, L.; Wei, L.; Wei, H.; Zhang, Y. The NH3 sensing properties of gas sensors based on aniline reduced graphene oxide. Synth. Met. 2013, 185–186, 25–30. [Google Scholar] [CrossRef]
- Zhao, F.G.; Zhao, G.; Liu, X.H.; Ge, C.W.; Wang, J.T.; Li, B.L.; Wang, Q.G.; Li, W.S.; Chen, Q.Y. Fluorinated graphene: Facile solution preparation and tailorable properties by fluorine-content tuning. J. Mater. Chem. A 2014, 2, 8782–8789. [Google Scholar] [CrossRef]
- Chen, A.; Shah, B. Electrochemical sensing and biosensing based on square wave voltammetry. Anal. Methods 2013, 5, 2158. [Google Scholar] [CrossRef]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, D.P.; Li, M.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayanan, V.; Zhang, Y.; Yoshihara, S.; Shirakashi, T. Voltammetric assay of naproxen in pharmaceutical formulations using boron-doped diamond electrode. Electroanalysis 2005, 17, 925–932. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-S.; Kim, K.H.; Kim, M.-J.; Lee, Y.-S. NH 3 gas sensing properties of a gas sensor based on fluorinated graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 104–109. [Google Scholar] [CrossRef]
- Lach, J.; Szymonik, A. Adsorption of naproxen sodium from aqueous solutions on commercial activated carbons. J. Ecol. Eng. 2019, 20, 241–251. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]









| GO-Based Nanomaterials | Carbon Atomic % | Oxygen Atomic % | Heteroatom Atomic % |
|---|---|---|---|
| GO | 69.11 | 30.89 | |
| TrGO | 84.61 | 15.39 | |
| B-rGO | 64.92 | 23.15 | 11.93 |
| F-GO | 69.36 | 29.97 | 0.67 |
| N-rGO | 86.91 | 10.12 | 2.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen. Sensors 2020, 20, 1252. https://0-doi-org.brum.beds.ac.uk/10.3390/s20051252
Qian L, Thiruppathi AR, Elmahdy R, van der Zalm J, Chen A. Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen. Sensors. 2020; 20(5):1252. https://0-doi-org.brum.beds.ac.uk/10.3390/s20051252
Chicago/Turabian StyleQian, Lanting, Antony Raj Thiruppathi, Reem Elmahdy, Joshua van der Zalm, and Aicheng Chen. 2020. "Graphene-Oxide-Based Electrochemical Sensors for the Sensitive Detection of Pharmaceutical Drug Naproxen" Sensors 20, no. 5: 1252. https://0-doi-org.brum.beds.ac.uk/10.3390/s20051252