Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flexible-Circuit Design
2.3. Electrode Fabrication
2.4. Membrane-Cocktail Fabrication
2.5. Measurement of Sensing Capabilities
3. Results and Discussion
3.1. Wireless Flexible Sodium Sensor for Sodium Detection in Saliva
3.2. Design and Fabrication of Film ISE
3.3. Characterization of CB/Ecoflex Composite ISE
3.3.1. Effect of Insulating Layer
3.3.2. Effect of Membrane Ingredient
3.4. Sensor-Performance Characterization with Mechanical Bending
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Matzeu, G.; O’Quigley, C.; McNamara, E.; Zuliani, C.; Fay, C.; Glennon, T.; Diamond, D. An integrated sensing and wireless communications platform for sensing sodium in sweat. Anal. Methods 2016, 8, 64–71. [Google Scholar] [CrossRef]
- Ainla, A.; Mousavi, M.P.S.; Tsaloglou, M.-N.; Redston, J.; Bell, J.G.; Fernández-Abedul, M.T.; Whitesides, G.M. Open-source potentiostat for wireless electrochemical detection with smartphones. Anal. Chem. 2018, 90, 6240–6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Howe, C.; Mishra, S.; Lee, D.S.; Mahmood, M.; Piper, M.; Kim, Y.; Tieu, K.; Byun, H.-S.; Coffey, J.P. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl. Acad. Sci. USA 2018, 115, 5377–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshimura, H.; Miyoshi, M.; Matsumoto, S.; Fujimoto, T.; Yamamoto, Y. Reflection of salt concentrations of blood upon those of saliva. Jpn. J. Physiol. 1963, 13, 523–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labat, C.; Thul, S.; Pirault, J.; Temmar, M.; Thornton, S.N.; Benetos, A.; Bäck, M. Differential associations for salivary sodium, potassium, calcium, and phosphate levels with carotid intima media thickness, heart rate, and arterial stiffness. Dis. Markers 2018, 2018, 3152146. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Marson, F.A.L.; Mendonça, R.M.H.; Bertuzzo, C.S.; Paschoal, I.A.; Ribeiro, J.D.; Ribeiro, A.F.; Levy, C.E. Chloride and sodium ion concentrations in saliva and sweat as a method to diagnose cystic fibrosis. J. Pediatr. 2019, 95, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Wardak, C. Solid contact cadmium ion-selective electrode based on ionic liquid and carbon nanotubes. Sens. Actuators B Chem. 2015, 209, 131–137. [Google Scholar] [CrossRef]
- Rius-Ruiz, F.X.; Crespo, G.A.; Bejarano-Nosas, D.; Blondeau, P.; Riu, J.; Rius, F.X. Potentiometric strip cell based on carbon nanotubes as transducer layer: Toward low-cost decentralized measurements. Anal. Chem. 2011, 83, 8810–8815. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zou, X.U.; Stein, A.; Bühlmann, P. Ion-selective electrodes with colloid-imprinted mesoporous carbon as solid contact. Anal. Chem. 2014, 86, 7111–7118. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Bao, C.; Hausmann, M.; Siqueira, G.; Zimmermann, T.; Kim, W.S. 3D printed disposable wireless ion sensors with biocompatible cellulose composites. Adv. Electron. Mater. 2019, 5, 1800778. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, T.; Xu, Z.; Hughes, E.; Qian, F.; Lee, M.; Fan, Y.; Lei, Y.; Brückner, C.; Li, B. Real-time in situ monitoring of nitrogen dynamics in wastewater treatment processes using wireless, solid-state, and ion-selective membrane sensors. Environ. Sci. Technol. 2019, 53, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.-H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable sweatband sensor platform based on gold nanodendrite array as efficient solid contact of ion-selective electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef] [PubMed]
- Papp, S.; Bojtár, M.; Gyurcsányi, R.E.E.; Lindfors, T. Potential reproducibility of potassium-selective electrodes having perfluorinated alkanoate side-chain functionalized poly (3, 4-ethylenedioxytiophene) as hydrophobic solid contact. Anal. Chem. 2019, 91, 9111–9118. [Google Scholar] [CrossRef] [PubMed]
- Kałuża, D.; Jaworska, E.; Mazur, M.; Maksymiuk, K.; Michalska, A. Multiwalled carbon nanotubes–poly(3-octylthiophene-2,5-diyl) nanocomposite transducer for ion-selective electrodes: Raman spectroscopy insight into the transducer/membrane interface. Anal. Chem. 2019, 91, 9010–9017. [Google Scholar] [CrossRef] [PubMed]
- Boeva, Z.A.; Lindfors, T. Few-layer graphene and polyaniline composite as ion-to-electron transducer in silicone rubber solid-contact ion-selective electrodes. Sens. Actuators B Chem. 2016, 224, 624–631. [Google Scholar] [CrossRef]
- Javanbakht, M.; Badiei, A.; Ganjali, M.R.; Norouzi, P.; Hasheminasab, A.; Abdouss, M. Use of organofunctionalized nanoporous silica gel to improve the lifetime of carbon paste electrode for determination of copper(II) ions. Anal. Chim. Acta 2007, 601, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, E.; Naitana, M.L.; Stelmach, E.; Pomarico, G.; Wojciechowski, M.; Bulska, E.; Maksymiuk, K.; Paolesse, R.; Michalska, A. Introducing cobalt(II) porphyrin/cobalt(III) corrole containing transducers for improved potential reproducibility and performance of all-solid-state ion-selective electrodes. Anal. Chem. 2017, 89, 7107–7114. [Google Scholar] [CrossRef] [PubMed]
- Khani, H.; Rofouei, M.K.; Arab, P.; Gupta, V.K.; Vafaei, Z. Multi-walled carbon nanotubes-ionic liquid-carbon paste electrode as a super selectivity sensor: Application to potentiometric monitoring of mercury ion(II). J. Hazard. Mater. 2010, 183, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Yin, T.; Qin, W. A simple approach for fabricating solid-contact ion-selective electrodes using nanomaterials as transducers. Anal. Chim. Acta 2015, 853, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, W.; Su, B. Highly hydrophobic solid contact based on graphene-hybrid nanocomposites for all solid state potentiometric sensors with well-formulated phase boundary potentials. J. Electroanal. Chem. 2015, 740, 21–27. [Google Scholar] [CrossRef]
- Topcu, C.; Lacin, G.; Yilmaz, V.; Coldur, F.; Caglar, B.; Cubuk, O.; Isildak, I. Electrochemical determination of copper(II) in water samples using a novel ion-selective electrode based on a graphite oxide–imprinted polymer composite. Anal. Lett. 2018, 51, 1890–1910. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Qin, Y.; Zhang, Y. Single-piece solid-contact ion-selective electrodes with polymer–carbon nanotube composites. Sens. Actuators B Chem. 2010, 148, 166–172. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, Y.; Zhang, Y. Preparation of all solid-state potentiometric ion sensors with polymer-CNT composites. Electrochem. Commun. 2009, 11, 1684–1687. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Ying, Y.; Wu, J. Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Yoon, Y.J.; Park, I. Ultra-stretchable and skin-mountable strain sensors using carbon nanotubes–Ecoflex nanocomposites. Nanotechnology 2015, 26, 375501. [Google Scholar] [CrossRef] [PubMed]
- Cholleti, E.R.; Stringer, J.; Assadian, M.; Battmann, V.; Bowen, C.; Aw, K. Highly stretchable capacitive sensor with printed carbon black electrodes on barium titanate elastomer composite. Sensor 2018, 19, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumbay Yildiz, S.; Mutlu, R.; Alici, G. Fabrication and characterisation of highly stretchable elastomeric strain sensors for prosthetic hand applications. Sens. Actuators A Phys. 2016, 247, 514–521. [Google Scholar] [CrossRef]
- Shintake, J.; Piskarev, E.; Jeong, S.H.; Floreano, D. Ultrastretchable strain sensors using carbon black-filled elastomer composites and comparison of capacitive versus resistive sensors. Adv. Mater. Technol. 2018, 3, 1700284. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Assadian, M.; Ramezani, M.; Aw, K.C. Printing of soft stretch sensor from carbon black composites. Proceedings 2018, 2, 732. [Google Scholar] [CrossRef] [Green Version]
- Giffney, T.; Xie, M.; Sartelet, M.; Aw, K.C. Vapor phase polymerization of PEDOT on silicone rubber as flexible large strain sensor. Aims Mater. Sci. 2015, 2, 414–424. [Google Scholar]
- Wu, W.; Wu, J.; Kim, J.-H.; Lee, N.Y. Instantaneous room temperature bonding of a wide range of non-silicon substrates with poly(dimethylsiloxane) (PDMS) elastomer mediated by a mercaptosilane. Lab. A Chip 2015, 15, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, S.; Bühlmann, P.; Pretsch, E.; Rusterholz, B.; Umezawa, Y. Cationic or anionic sites? Selectivity optimization of ion-selective electrodes based on charged ionophores. Anal. Chem. 2000, 72, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Kim, Y.-S.; Intarasirisawat, J.; Kwon, Y.-T.; Lee, Y.; Mahmood, M.; Lim, H.-R.; Herbert, R.; Yu, K.J.; Ang, C.S. Soft, wireless periocular wearable electronics for real-time detection of eye vergence in a virtual reality toward mobile eye therapies. Sci. Adv. 2020, 6, eaay1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, M.; Mzurikwao, D.; Kim, Y.-S.; Lee, Y.; Mishra, S.; Herbert, R.; Duarte, A.; Ang, C.S.; Yeo, W.-H. Fully portable and wireless universal brain–machine interfaces enabled by flexible scalp electronics and deep learning algorithm. Nat. Mach. Intell. 2019, 1, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Guinovart, T.; Crespo, G.A.; Rius, F.X.; Andrade, F.J. A reference electrode based on polyvinyl butyral (PVB) polymer for decentralized chemical measurements. Anal. Chim. Acta 2014, 821, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Iyer, E.M.; Malik, H. Relative changes in salivary sodium and potassium in relation to exposure to high G stress. Med. J. Armed Forces India 1994, 50, 261–265. [Google Scholar] [CrossRef] [Green Version]
- White, A.G.; Entmacher, P.S.; Rubin, G.; Leiter, L. Physiological and pharmacological regulation of human salivary electrolyte concentrations; with a discussion of electrolyte concentrations of some other exocrine secretions. J. Clin. Investig. 1955, 34, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J. Potential stability of all-solid-state ion-selective electrodes using conducting polymers as ion-to-electron transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Z.; Fierke, M.A.; Stein, A.; Bühlmann, P. Ion-selective electrodes with three-dimensionally ordered macroporous carbon as the solid contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Khaled, E.; Hassan, H.; Mohamed, G.G.; Ragab, F.A.; Seleim, A. β-Cyclodextrin-based potentiometric sensors for flow-injection determination of acetylcholines. Int. J. Electrochem. Sci. 2010, 5, 448–458. [Google Scholar]
- Al-Alamein, A.M.A.; Kamel, M.S.; El-Alamin, M.M.A.; Khaled, E. Novel pioglitazone nanomaterial based screen printed sensors. Int. J. Electrochem. Sci. 2015, 10, 2400–2412. [Google Scholar]
- Chen, X.; Hu, Q.; Chen, S.; Netzer, N.L.; Wang, Z.; Zhang, S.-L.; Zhang, Z. Multiplexed analysis of molecular and elemental ions using nanowire transistor sensors. Sens. Actuators B Chem. 2018, 270, 89–96. [Google Scholar] [CrossRef]
- Luboch, E.; Jeszke, M.; Szarmach, M.; Łukasik, N. New bis(azobenzocrown)s with dodecylmethylmalonyl linkers as ionophores for sodium selective potentiometric sensors. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 323–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moya, A.; Illa, X.; Gimenez, I.; Lazo-Fernandez, Y.; Villa, R.; Errachid, A.; Gabriel, G. Miniaturized multiparametric flexible platform for the simultaneous monitoring of ionic: Application in real urine. Sens. Actuators B Chem. 2018, 255, 2861–2870. [Google Scholar] [CrossRef] [Green Version]
- Paczosa-Bator, B.; Pięk, M.; Piech, R. Application of nanostructured TCNQ to potentiometric ion-selective K+ and Na+ electrodes. Anal. Chem. 2015, 87, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.E.; Isildak, I.; Nechifor, A.C.; Cristache, C.M.; Enachescu, M. New sensor based on membranes with magnetic nano-inclusions for early diagnosis in periodontal disease. Biosens. Bioelectron. 2018, 102, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, Y.; Bühlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (technical report). Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Kubala, E.; Strzelecka, P.; Grzegocka, M.; Lietz-Kijak, D.; Gronwald, H.; Skomro, P.; Kijak, E. A review of selected studies that determine the physical and chemical properties of saliva in the field of dental treatment. Biomed. Res. Int. 2018, 2018, 6572381. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Kim, Y.; Jierry, L.; Hemmerle, J.; Boulmedais, F.; Schaaf, P.; Pronkin, S.; Kotov, N.A. Electrochemistry on stretchable nanocomposite electrodes: Dependence on strain. ACS Nano 2018, 12, 9223–9232. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, K.; Chen, D.; Shen, G. Wearable sweat monitoring system with integrated micro-supercapacitors. Nano Energy 2019, 58, 624–632. [Google Scholar] [CrossRef]
- Roy, S.; David-Pur, M.; Hanein, Y. Carbon nanotube-based ion selective sensors for wearable applications. ACS Appl. Mater. Interfaces 2017, 9, 35169–35177. [Google Scholar] [CrossRef] [PubMed]
- Stekolshchikova, A.A.; Radaev, A.V.; Orlova, O.Y.; Nikolaev, K.G.; Skorb, E.V. Thin and flexible ion sensors based on polyelectrolyte multilayers assembled onto the carbon adhesive tape. ACS Omega 2019, 4, 15421–15427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Wang, S.; Bai, Y.; Yang, X.; Liu, L.; Li, L.; Lu, Q.; Li, T.; Zhang, T. Highly stretchable potentiometric ion sensor based on surface strain redistributed fiber for sweat monitoring. Talanta 2020, 214, 120869. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Ferré, J.; Guinovart, T.; Andrade, F.J. Wearable potentiometric sensors based on commercial carbon fibres for monitoring sodium in sweat. Electroanalysis 2016, 28, 1267–1275. [Google Scholar] [CrossRef]





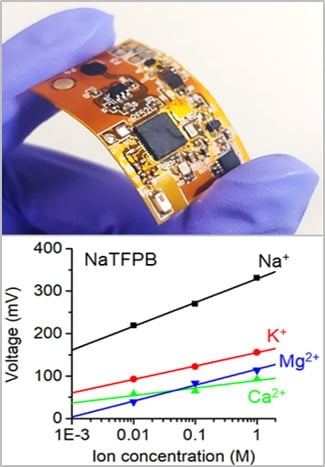
| Interfering Ion, J | KTClPB | NaTFPB | ||
|---|---|---|---|---|
| Sensitivity (mV/decade) | KpotNa+ J | Sensitivity (mV/decade) | KpotNa+ J | |
| K+ | 47.5 | −0.6 | 31.6 | −3.0 |
| Mg2+ | 21.1 | −6.6 | 37.5 | −6.9 |
| Ca2+ | 3.9 | −7.7 | 17.7 | −7.6 |
| Na+ | 52.9 | 0 | 56.1 | 0 |
| Reference | Substrate | Flexible/Wireless | Sensitivity (mV/decade) (1) | Selectivity (KpotNa+ K+) | Lifetime |
|---|---|---|---|---|---|
| This work | PI | Yes/yes | 56.1 | –3.0 | 3 weeks |
| [50] | PET | Yes/yes | 0.031 nF/mM (2) | Not available | Not available |
| [51] | PDMS | Yes/no | 58 | Not available | <1 h |
| [52] | Tape | Yes/no | 56.2 | Not available | Not available |
| [53] | Paper | Yes/no | 55.7 | Not available | Not available |
| [54] | Fiber | Yes/no | 55.1 | Not available | 4 weeks |
| [55] | Plastic | Some/no | 55.9 | −2.2 | Not available |
| [12] | Wafer | Some/no | 56.6 | −2.5 | 2 months |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-R.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Kwon, Y.-T.; Lee, Y.; Lee, S.M.; Yeo, W.-H. Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium. Sensors 2020, 20, 3297. https://0-doi-org.brum.beds.ac.uk/10.3390/s20113297
Lim H-R, Kim Y-S, Kwon S, Mahmood M, Kwon Y-T, Lee Y, Lee SM, Yeo W-H. Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium. Sensors. 2020; 20(11):3297. https://0-doi-org.brum.beds.ac.uk/10.3390/s20113297
Chicago/Turabian StyleLim, Hyo-Ryoung, Yun-Soung Kim, Shinjae Kwon, Musa Mahmood, Young-Tae Kwon, Yongkuk Lee, Soon Min Lee, and Woon-Hong Yeo. 2020. "Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium" Sensors 20, no. 11: 3297. https://0-doi-org.brum.beds.ac.uk/10.3390/s20113297