Recent Advances in Electrochemical Sensors and Biosensors for Detecting Bisphenol A
Abstract
:1. Introduction
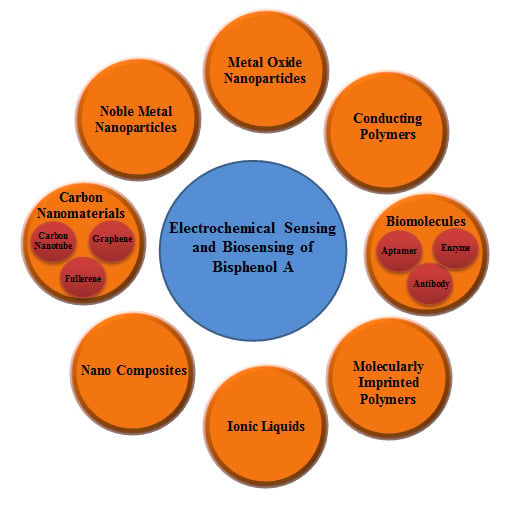
2. Electrochemical Sensing and Biosensing of BPA
2.1. Carbon Nanomaterial-Based Electrochemical Sensors
2.1.1. CNT-Based Electrochemical Sensors
2.1.2. Gr-Based Electrochemical Sensors
2.1.3. Fullerene-Based Electrochemical Sensors
2.2. Noble Metal NP-Based Electrochemical Sensors
2.3. Metal Oxide NP-Based Electrochemical Sensors
2.4. Polymer-Based Electrochemical Sensors
2.5. Nanocomposite-Based Electrochemical Sensors
2.6. Ionic Liquid (IL)-Based Electrochemical Sensors
2.7. Molecularly Imprinted Polymer-Based Electrochemical Sensors
2.8. Biomolecule-Based Electrochemical Biosensors
2.8.1. Aptamer-Based Electrochemical Biosensors
2.8.2. Enzyme-Based Electrochemical Biosensors
2.8.3. Antibody-Based Electrochemical Biosensors
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr.Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef] [Green Version]
- Ragavan, K.V.; Rastogi, N.K.; Thakur, M.S. Sensors and biosensors for analysis of bisphenol-A. TrAC Trend. Anal. Chem. 2013, 52, 248–260. [Google Scholar] [CrossRef]
- Chang, H.S.; Choo, K.H.; Lee, B.; Choi, S.J. The methods of identification, analysis, and removal of endocrine disrupting compounds (EDCs) in water. J. Hazard. Mater. 2009, 172, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Varmira, K.; Saed-Mocheshi, M.; Jalalvand, A.R. Electrochemical sensing and bio-sensing of bisphenol A and detection of its damage to DNA: A comprehensive review. Sens. Bio-Sensing Res. 2017, 15, 17–33. [Google Scholar] [CrossRef]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef]
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006, 254, 179–186. [Google Scholar] [CrossRef]
- Zou, J.; Zhao, G.Q.; Teng, J.; Liu, Q.; Jiang, X.Y.; Jiao, F.P.; Yu, J.G. Highly sensitive detection of bisphenol A in real water samples based on in-situ assembled graphenenanoplatelets and gold nanoparticles composite. Microchem. J. 2019, 145, 693–702. [Google Scholar] [CrossRef]
- Basheer, C.; Lee, H.K. Analysis of endocrine disrupting alkylphenols, chlorophenols and bisphenol-A using hollow fiber-protected liquid-phase microextraction coupled with injection port-derivatization gas chromatography–mass spectrometry. J. Chromatogr. A 2004, 1057, 163–169. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Zhu, X. Solid phase extraction of bisphenol A using magnetic core-shell (Fe3O4@SiO2) nanoparticles coated with an ionic liquid, and its quantitation by HPLC. Microchim. Acta 2016, 183, 1315–1321. [Google Scholar] [CrossRef]
- Cao, X.; Shen, F.; Zhang, M.; Bie, J.; Liu, X.; Luo, Y.; Guo, J.; Sun, C. Facile synthesis of chitosan-capped ZnS quantum dots as an eco-friendly fluorescence sensor for rapid determination of bisphenol A in water and plastic samples. RSC Adv. 2014, 4, 16597–16606. [Google Scholar] [CrossRef] [Green Version]
- Santana, E.R.; de Lima, C.A.; Piovesan, J.V.; Spinelli, A. An original ferroferric oxide and gold nanoparticles-modified glassy carbon electrode for the determination of bisphenol A. Sens. Actuators B Chem. 2017, 240, 487–496. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mahmoudi-Moghaddam, H.; Tajik, S. Voltammetricdetermination of bisphenola in water and juice using a lanthanum (III)-doped cobalt (II, III) nanocubemodified carbon screen-printed electrode. Anal. Lett. 2019, 52, 1432–1444. [Google Scholar] [CrossRef]
- Lu, C.; Li, J.; Yang, Y.; Lin, J.M. Determination of bisphenol A based on chemiluminescence from gold (III)–peroxymonocarbonate. Talanta 2010, 82, 1576–1580. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Han, X. Chemical sensors for environmental pollutant determination. In Chemical, Gas, and Biosensors for Internet of Things and Related Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 147–160. [Google Scholar]
- Zhang, K.; Lee, T.H.; Choi, M.-J.; Rajabi-Abhari, A.; Choi, S.; Choi, K.S.; Varma, R.S.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M. Electrochemical activity of samarium on starch-derived porous carbon: Rechargeable Li-and Al-ion batteries. Nano Converg. 2020, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Kirlikovali, K.O.; Le, Q.V.; Jin, Z.; Varma, R.S.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Extended metal-organic frameworks on diverse supports as electrode nanomaterials for electrochemical energy storage. ACS Appl. Nano Mater. 2020, 3, 3964–3990. [Google Scholar] [CrossRef]
- Zhang, K.; Lee, T.H.; Khalilzadeh, M.A.; Varma, R.S.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M. Rendering redox reactions of cathodes in Li-ion capacitors enabled by lanthanides. ACS Omega 2020, 5, 1634–1639. [Google Scholar] [CrossRef]
- Zhang, K.; Kirlikovali, K.O.; Varma, R.S.; Jin, Z.; Jang, H.W.; Farha, O.K.; Shokouhimehr, M. Covalent organic frameworks: Emerging organic solid materials for energy and electrochemical applications. ACS Appl. Mater. Inter. 2020. [CrossRef]
- Buzid, A.; McGlacken, G.P.; Glennon, J.D.; Luong, J.H. Electrochemical sensing of biotin using Nafion-modified boron-doped diamond electrode. ACS Omega 2018, 3, 7776–7782. [Google Scholar] [CrossRef]
- Beitollahi, H.; Karimi-Maleh, H.; Khabazzadeh, H. Nanomolar and selective determination of epinephrine in the presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-oxo-3-phenyl-3, 4-dihydro-quinazolinyl)-N′-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Celiesiute, R.; Ramanaviciene, A.; Shirsat, M.D.; Ramanavicius, A. EDTA_PANI/SWCNTs nanocomposite modified electrode for electrochemical determination of copper (II), lead (II) and mercury (II) ions. Electrochim. Acta 2018, 259, 930–938. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H. First report for simultaneous determination of methyldopa and hydrochlorothiazide using a nanostructured based electrochemical sensor. J. Electroanal. Chem. 2013, 704, 137–144. [Google Scholar] [CrossRef]
- Liu, T.; Meng, F.; Cheng, W.; Sun, H.; Luo, Y.; Tang, Y.; Miao, P. Preparation of a peptide-modified electrode for capture and voltammetric determination of endotoxin. ACS Omega 2017, 2, 2469–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi-Moghaddam, H.; Beitollahi, H.; Tajik, S.; Soltani, H. Fabrication of a nanostructure based electrochemical sensor for voltammetric determination of epinephrine, uric acid and folic acid. Electroanalysis 2015, 27, 2620–2628. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Atty, S.A.; Yehia, A.M.; Foster, C.W.; Banks, C.E.; Allam, N.K. Electrochemical determination of the serotonin reuptake inhibitor, dapoxetine, using cesium-gold nanoparticles. ACS Omega 2017, 2, 6628–6635. [Google Scholar] [CrossRef] [PubMed]
- Tajik, S.; Taher, M.A.; Beitollahi, H. Simultaneous determination of droxidopa and carbidopa using a carbon nanotubes paste electrode. Sens. Actuators B Chem. 2013, 188, 923–930. [Google Scholar] [CrossRef]
- Yin, H.; Cui, L.; Ai, S.; Fan, H.; Zhu, L. Electrochemical determination of bisphenol A at Mg–Al–CO3 layered double hydroxide modified glassy carbon electrode. Electrochim. Acta 2010, 55, 603–610. [Google Scholar] [CrossRef]
- Ghanam, A.; Lahcen, A.A.; Amine, A. Electroanalytical determination of Bisphenol A: Investigation of electrode surface fouling using various carbon materials. J. Electroanal. Chem. 2017, 789, 58–66. [Google Scholar] [CrossRef]
- Vega, D.; Agüí, L.; González-Cortés, A.; Yáñnez-Sedño, P.; Pingarrón, J.M. Electrochemical detection of phenolic estrogenic compounds at carbon nanotube-modified electrodes. Talanta 2007, 71, 1031–1038. [Google Scholar] [CrossRef]
- Tajik, S.; Taher, M.A.; Beitollahi, H.; Torkzadeh-Mahani, M. Electrochemical determination of the anticancer drug taxol at a ds-DNA modified pencil-graphite electrode and its application as a label-free electrochemical biosensor. Talanta 2015, 134, 60–64. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Beitollahi, H.; Zaimbashi, R.; Tajik, S.; Rezapour, M.; Larijani, B. Voltammetric determination of dopamine using glassy carbon electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 2018, 13, 2519–2529. [Google Scholar] [CrossRef]
- Shankar, S.S.; Shereema, R.M.; Ramachandran, V.; Sruthi, T.V.; Kumar, V.S.; Rakhi, R.B. Carbon quantum dot-modified carbon paste electrode-based sensor for selective and sensitive determination of adrenaline. ACS Omega 2019, 4, 7903–7910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, H.; Beitollahi, H.; Hatefi-Mehrjardi, A.H.; Tajik, S.; Torkzadeh-Mahani, M. Voltammetric determination of glutathione using a modified single walled carbon nanotubes paste electrode. Anal. Bioanal. Electrochem. 2014, 6, 67–79. [Google Scholar]
- Venu, M.; Venkateswarlu, S.; Reddy, Y.V.M.; Seshadri Reddy, A.; Gupta, V.K.; Yoon, M.; Madhavi, G. Highly sensitive electrochemical sensor for anticancer drug by a zirconia nanoparticle-decorated reduced graphene oxide nanocomposite. ACS Omega 2018, 3, 14597–14605. [Google Scholar] [CrossRef] [Green Version]
- Tajik, S.; Taher, M.A.; Beitollahi, H. First report for electrochemical determination of levodopa and cabergoline: Application for determination of levodopa and cabergoline in human serum, urine and pharmaceutical formulations. Electroanalysis 2014, 26, 796–806. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Gui, R.; Jin, H.; Xia, Y. Carbon nanomaterials-based electrochemical aptasensors. Biosens. Bioelectron. 2016, 79, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhu, Z.; Du, D.; Lin, Y. Nanomaterial-based electrochemical biosensors for food safety. J. Electroanal. Chem. 2016, 781, 147–154. [Google Scholar] [CrossRef]
- Li, J.; Kuang, D.; Feng, Y.; Zhang, F.; Liu, M. Voltammetric determination of bisphenol A in food package by a glassy carbon electrode modified with carboxylated multi-walled carbon nanotubes. Microchim. Acta 2011, 172, 379–386. [Google Scholar] [CrossRef]
- Gao, Y.; Cao, Y.; Yang, D.; Luo, X.; Tang, Y.; Li, H. Sensitivity and selectivity determination of bisphenol A using SWCNT–CD conjugate modified glassy carbon electrode. J. Hazard. Mater. 2012, 199, 111–118. [Google Scholar] [CrossRef]
- Cosio, M.S.; Pellicanò, A.; Brunetti, B.; Fuenmayor, C.A. A simple hydroxylated multi-walled carbon nanotubes modified glassy carbon electrode for rapid amperometric detection of bisphenol A. Sens. Actuators B Chem. 2017, 246, 673–679. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019, 23, e00064. [Google Scholar] [CrossRef]
- Ntsendwana, B.; Mamba, B.B.; Sampath, S.; Arotiba, O.A. Electrochemical detection of bisphenolA using graphene-modified glassy carbon electrode. Int. J. Electrochem. Sci. 2012, 7, 3501–3512. [Google Scholar]
- Mazloum-Ardakani, M.; Khoshroo, A.; Hosseinzadeh, L. Simultaneous determination of hydrazine and hydroxylamine based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sens. Actuators B Chem. 2015, 214, 132–137. [Google Scholar] [CrossRef]
- Rather, J.A.; De Wael, K. Fullerene-C60 sensor for ultra-high sensitive detection of bisphenol-A and its treatment by green technology. Sens. Actuators B Chem. 2013, 176, 110–117. [Google Scholar] [CrossRef]
- Pareek, V.; Bhargava, A.; Gupta, R.; Jain, N.; Panwar, J. Synthesis and applications of noble metal nanoparticles: A review. Adv. Sci. Eng. Med. 2017, 9, 527–544. [Google Scholar] [CrossRef]
- Chen, W.Y.; Mei, L.P.; Feng, J.J.; Yuan, T.; Wang, A.J.; Yu, H. Electrochemical determination of bisphenol A with a glassy carbon electrode modified with gold nanodendrites. Microchim. Acta 2015, 182, 703–709. [Google Scholar] [CrossRef]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef]
- Mohammadzadeh-Jahani, P.; Beitollahi, H.; Tajik, S.; Tashakkorian, H. Selective electrochemical determination of bisphenol A via a Fe3O4 NPs derivative-modified graphite screen-printed electrode. Int. J. Environ. Anal. Chem. 2019, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.; Ellis, H.; Yang, L.; Johansson, E.M.; Boschloo, G.; Vlachopoulos, N.; Hagfeldt, A.; Bergquist, J.; Shevchenko, D. Matrix-assisted laser desorption/ionization mass spectrometric analysis of poly (3, 4-ethylenedioxythiophene) in solid-state dye-sensitized solar cells: Comparison of in situ photoelectrochemical polymerization in aqueous micellar and organic media. Anal. Chem. 2015, 87, 3942–3948. [Google Scholar] [CrossRef]
- Mazzotta, E.; Malitesta, C.; Margapoti, E. Direct electrochemical detection of bisphenol A at PEDOT-modified glassy carbon electrodes. Anal. Bioanal. Chem. 2013, 405, 3587–3592. [Google Scholar] [CrossRef]
- Tian, K.; Zhang, Y.; Zhang, S.; Dong, Y. Electrogeneratedchemiluminescence of ZnO/MoS2 nanocomposite and its application for cysteine detection. J. Electrochem. Soc. 2019, 166, H527–H533. [Google Scholar] [CrossRef]
- Fan, H.; Li, Y.; Wu, D.; Ma, H.; Mao, K.; Fan, D.; Du, B.; Li, H.; Wei, Q. Electrochemical bisphenol A sensor based on N-doped graphene sheets. Anal. Chim. Acta 2012, 711, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Yaman, Y.T.; Abaci, S. Sensitive adsorptive voltammetric method for determination of bisphenol A by gold nanoparticle/polyvinylpyrrolidone-modified pencil graphite electrode. Sensors 2016, 16, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Si, Y.; Li, C.; Zhang, K. Bisphenol a electrochemical sensor based on multi-walled carbon nanotubes/polythiophene/ptnanocomposites modified electrode. Anal. Methods 2016, 8, 3333–3338. [Google Scholar] [CrossRef]
- Messaoud, N.B.; Ghica, M.E.; Dridi, C.; Ali, M.B.; Brett, C.M. Electrochemical sensor based on multiwalled carbon nanotube and gold nanoparticle modified electrode for the sensitive detection of bisphenol A. Sens. Actuators B Chem. 2017, 253, 513–522. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Yan, B.; Zhang, H. An electrochemical sensor for the determination of bisphenol A using glassy carbon electrode modified with reduced graphene oxide-silver/poly-L-lysine nanocomposites. J. Electroanal. Chem. 2017, 805, 39–46. [Google Scholar] [CrossRef]
- Hao, Y.U.; Xiao, F.E.N.G.; Xiao-Xia, C.H.E.N.; Jin-Li, Q.I.A.O.; Xiao-Ling, G.A.O.; Na, X.U.; Lou-Jun, G.A.O. Electrochemical determination of bisphenol A on a glassy carbon electrode modified with gold nanoparticles loaded on reduced graphene oxide-multi walled carbon nanotubes composite. Chinese J. Anal. Chem. 2017, 45, 713–720. [Google Scholar]
- Abo-Hamad, A.; AlSaadi, M.A.; Hayyan, M.; Juneidi, I.; Hashim, M.A. Ionic liquid-carbon nanomaterial hybrids for electrochemical sensor applications: A review. Electrochim. Acta 2016, 193, 321–343. [Google Scholar] [CrossRef]
- Chen, X.; Ren, T.; Ma, M.; Wang, Z.; Zhan, G.; Li, C. Voltammetric sensing of bisphenol A based on a single-walled carbon nanotubes/poly {3-butyl-1-[3-(N-pyrrolyl) propyl] imidazolium ionic liquid} composite film modified electrode. Electrochim. Acta 2013, 111, 49–56. [Google Scholar] [CrossRef]
- Najafi, M.; Khalilzadeh, M.A.; Karimi-Maleh, H. A new strategy for determination of bisphenol A in the presence of Sudan I using a ZnO/CNTs/ionic liquid paste electrode in food samples. Food Chem. 2014, 158, 125–131. [Google Scholar] [CrossRef]
- Ahmad, O.S.; Bedwell, T.S.; Esen, C.; Garcia-Cruz, A.; Piletsky, S.A. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019, 37, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical determination of bisphenolA in plastic bottled drinking water and canned beverages using a molecularly imprinted chitosan–graphene composite film modified electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An electrochemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sens. Actuators B Chem. 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Hierlemann, A.; Brand, O.; Hagleitner, C.; Baltes, H. Microfabrication techniques for chemical/biosensors. Proc. IEEE 2003, 91, 839–863. [Google Scholar] [CrossRef] [Green Version]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, J.; Li, D.; Li, Y. An electrochemical aptasensor based on gold nanoparticles dotted graphene modified glassy carbon electrode for label-free detection of bisphenol A in milk samples. Food Chem. 2014, 162, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Deiminiat, B.; Rounaghi, G.H.; Arbab-Zavar, M.H.; Razavipanah, I. A novel electrochemical aptasensor based on f-MWCNTs/Au NPs nanocomposite for label-free detection of bisphenol A. Sens. Actuators B Chem. 2017, 242, 158–166. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ansari, R.; Nodehi, M.; Razavipanah, I.; Veisi, H. Voltammetricaptasensor for bisphenol A based on the use of a MWCNT/Fe3O4@gold nanocomposite. Microchim. Acta 2018, 185, 320. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants–trends and perspective. TrAC Trend. Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Deng, Y.; Liu, G.; Hou, X.; Huang, Y.; Li, C. Enhanced biosensing of bisphenol A using a nanointerface based on tyrosinase/reduced graphene oxides functionalized with ionic liquid. Electroanalysis 2016, 28, 96–102. [Google Scholar] [CrossRef]
- Kunene, K.; Sabela, M.; Kanchi, S.; Bisetty, K. High performance electrochemical biosensor for bisphenol a using screen printed electrodes modified with multiwalled carbon nanotubes functionalized with silver-doped zinc oxide. Waste Biomass Valori. 2020, 11, 1085–1096. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, L.; He, L.; Liu, N.; Piao, Y. Electrochemical Enzyme Biosensor Bearing Biochar Nanoparticle as Signal Enhancer for Bisphenol A Detection in Water. Sensors 2019, 19, 1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadir, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2014, 44, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, X.; Zheng, S. A novel and label-free immunosensor for bisphenol A using rutin as the redox probe. Talanta 2016, 160, 241–246. [Google Scholar] [CrossRef]




| Ref. | Linear Range | Detection Limit | Method | Electrochemical Sensor |
|---|---|---|---|---|
| [39] | 10–104 nM | 5.0 nM | Voltammetry | c-MWCNTs/GCE |
| [40] | 10.8 nM–18.5 µM | 1.0 nM | Amperometry | SWCNT–â-CD/GCE |
| [41] | 1–24 µg L−1 | 0.81 µg L−1 | Amperometry | MWCNTs-OH/GCE |
| [43] | 5 × 10−8–1 × 10−6 M | 4.689 × 10−8 M | DPV | Gr/GCE |
| [45] | 74 nM−0.23 µM | 3.7 nM | SWV | Fullerene/GCE |
| [47] | 0.05–55.0 µM | 1.2 nM | DPV | 4-MBA/gold nano-dendrites/GCE |
| [49] | 0.03–700.0 µM | 0.01 µM | DPV | Fe3O4 NP-derivative/SPE |
| [51] | 90–410 µM | 55 µM | CV | PEDOT/GCE |
| 40–410 µM | 22 µM | Amperometry | ||
| [53] | 1.0 × 10−8–1.3 × 10−6 M | 5.0 × 10−9 M | Amperometry | CS/N-GrS/GCE |
| [54] | 0.03–1.10 µM | 1.0 nM | SWAdSV | AuNP/PVP/PGE |
| [55] | 5.0 × 10−8–4.0 × 10−7 M | 3.0 × 10−9 M | DPV | MWCNT/PTh/Pt/GCE |
| [56] | 0.01–0.7 µM | 4 nM | DPV | AuNP/MWCNT/GCE |
| [57] | 1–80 µM | 0.54 µM | DPV | rGO–Ag/PLL/GCE |
| [58] | 5.0 × 10−9−2.0 × 10−5 M | 1.0 × 10−9 M | DPV | AuNP–rGO–MWCNTs/GCE |
| [60] | 5.0 × 10−9–3.0 × 10−5 M | 1.0 × 10−9 M | DPV | SWCNTs/Poly-IL/GCE |
| [61] | 0.002–700 µM | 9.0 nM | SWV | ZnO/CNT/IL/CPE |
| [63] | 8.0−2.0 µM | 6.0 nM | Derivative Voltammetry | MIP/CS/Gr/ABPE |
| [64] | 0.1–50 µM | 0.04 µM | DPV | MIPPy/GQDs/GCE |
| [67] | 0.01–10 µM | 5 nM | DPV | AuNP/Gr/GCE |
| [68] | 0.1–10 nM | 0.05 nM | SWV | f-MWCNT/AuNP/Au |
| [69] | 0.1–8 nM | 0.03 nM | DPV | MWCNT/Fe3O4-SH@Au/ |
| aptamer/MCH/GCE | ||||
| [71] | 1.0 × 10−9–3. 8 × 10−5 M | 3.5 × 10−10 M | Amperometry | Tyr-DAPPT–rGO/GCE |
| [72] | 0.5–2.99 µM | 6.0 nM | DPV | Lac/Ag–ZnO/MWCNTs/CSPE |
| [73] | 0.02–10 µM | 3.18 nM | Amperometry | BCNP/Tyr/Nafion/GCE |
| [75] | 1.0 × 10−8–1. 0 × 10−6 M | 8.7 × 10−9 M | LSV | BSA/Anti-BPA/AuNP/MWCNT/GCE |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajik, S.; Beitollahi, H.; Nejad, F.G.; Zhang, K.; Le, Q.V.; Jang, H.W.; Kim, S.Y.; Shokouhimehr, M. Recent Advances in Electrochemical Sensors and Biosensors for Detecting Bisphenol A. Sensors 2020, 20, 3364. https://0-doi-org.brum.beds.ac.uk/10.3390/s20123364
Tajik S, Beitollahi H, Nejad FG, Zhang K, Le QV, Jang HW, Kim SY, Shokouhimehr M. Recent Advances in Electrochemical Sensors and Biosensors for Detecting Bisphenol A. Sensors. 2020; 20(12):3364. https://0-doi-org.brum.beds.ac.uk/10.3390/s20123364
Chicago/Turabian StyleTajik, Somayeh, Hadi Beitollahi, Fariba Garkani Nejad, Kaiqiang Zhang, Quyet Van Le, Ho Won Jang, Soo Young Kim, and Mohammadreza Shokouhimehr. 2020. "Recent Advances in Electrochemical Sensors and Biosensors for Detecting Bisphenol A" Sensors 20, no. 12: 3364. https://0-doi-org.brum.beds.ac.uk/10.3390/s20123364