Sensor Based on Molecularly Imprinted Polymer Membranes and Smartphone for Detection of Fusarium Contamination in Cereals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical and Analytical Methods
2.2.1. Synthesis of Cyclododecyl-2,4-Dihydroxybenzoate (CDHB)
2.2.2. Synthesis of 2-[(Pyrene-1-Carbonyl) Amino]Ethyl 2,4-Dihydroxynenzoate (PARA)
N-(2-hydroxyethyl)-pyrene-1-carboxamide (6)
2-[(Pyrene-1-carbonyl) amino]ethyl 2,4-dihydroxynenzoate (PARA)
2.2.3. Synthesis of Zearalenone-Selective MIP Membranes
2.3. Evaluation of Recognition Properties of Zearalenone-Selective Membranes
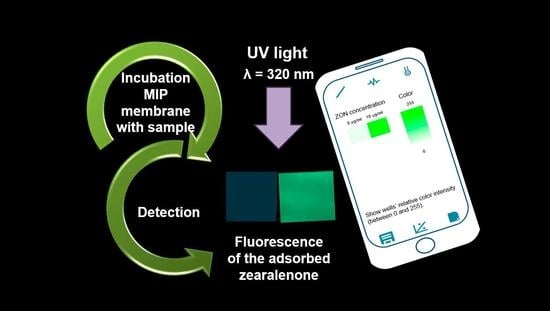
2.4. Smartphone Sensor Based on Zearalenone-Selective MIP Membranes
2.5. Preparation of Flour Sample Extracts for the Analysis
3. Results and Discussion
3.1. Synthesis of A Zearalenone Mimic Cyclododecyl Ester of 2,4-Dihydroxybenzoic Acid
3.2. Synthesis of the Zearalenone-Selective MIP Membranes and Their Application for the Development of Fluorescent Sensor Systems
3.3. Development of the Competitive Fluorescent Sensor Assay for Highly Sensitive Zearalenone Detection
3.4. Application of the Zearalenone-Selective Sensor System for Analysis of Cereal Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [Green Version]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef] [Green Version]
- Etienne, M.; Dourmad, J.Y. Effects of zearalenone or glucosinolates in the diet on reproduction in sows: A review. Livest. Prod. Sci. 1994, 40, 99–113. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D. Mycotoxins in spices and herbs-An update. Crit. Rev. Food Sci. Nutr. 2017, 57, 18–34. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Kyprianou, M. Commission regulation (EC) No1126/2007 of 28 September 2007 amendingregulation (EC)No1881/2006 setting maximum levels for certain contaminants in food stuffs as regards fusarium toxins in maize and maizeproducts. Off. J. Eur. Union 2007, 255, 14–17. [Google Scholar]
- European Commission. Commission regulation (EC) No1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, L364/5–L364/24. [Google Scholar]
- Ok, H.E.; Choi, S.W.; Kim, M.; Chun, H.S. HPLC and UPLC methods for the determination of zearalenone in noodles, cereal snacks and infant formula. Food Chem. 2014, 163, 252–257. [Google Scholar] [CrossRef]
- Huang, L.C.; Zheng, N.; Zheng, B.Q.; Wen, F.; Cheng, J.B.; Han, R.W.; Xu, X.M.; Li, S.L.; Wang, J.Q. Simultaneous determination of afltoxin M1, ochratoxin A, zearalenone and zearalenol in milk by UHPLC-MS/MS. Food Chem. 2014, 146, 242–249. [Google Scholar] [CrossRef]
- Songsermsakul, P.; Sontag, G.; Cichna-Markl, M.; Zentek, J.; Razzazi-Fazeli, E. Determination of zearalenone and its metabolites in urine, plasma, and faeces of horses by HPLC-APCI-MS. J. Chromatogr. B 2006, 843, 252–261. [Google Scholar] [CrossRef]
- Rodríguez-Carrasco, Y.; Moltó, J.C.; Berrada, H.; Manes, J. A survey of trichothecenes, zearalenone, and patulin in milled grain-based products using GC-MS/MS. Food Chem. 2014, 146, 212–219. [Google Scholar] [CrossRef]
- Urraca, J.L.; Marazuela, M.D.; Merino, E.R.; Orellana, G.; Moreno-Bondi, M.C. Molecularly imprinted polymers with a streamlined mimic for zearalenone analysis. J. Chromatogr. A 2006, 1116, 127–134. [Google Scholar] [CrossRef]
- Navarro-Villoslada, F.; Urraca, J.L.; Moreno-Bondi, M.C.; Orellana, G. Zearalenone sensing with molecularly imprinted polymers and tailored fluorescent probes. Sens. Actuators B 2007, 121, 67–73. [Google Scholar] [CrossRef]
- Pei, S.C.; Lee, W.J.; Zhang, G.P.; Hu, X.F.; Eremin, S.A.; Zhang, L.J. Development of anti-zearalenone monoclonal antibody and detection of zearalenone in corn products from China by ELISA. Food Control 2013, 31, 65–70. [Google Scholar] [CrossRef]
- Liu, N.; Nie, D.; Zhao, Z.; Hu, X.F.; Eremin, S.A.; Zhang, L.J. Ultrasensitive immunoassays based on biotin-streptavidin amplified system for quantitative determination of family zearalenones. Food Control 2015, 57, 202–209. [Google Scholar] [CrossRef]
- Zhan, S.; Huang, X.; Chen, R.; Li, J.; Xiong, Y. Novel fluorescent ELISA for the sensitive detection of zearalenone based on H2O2-sensitive quantum dots for signal transduction. Talanta 2016, 158, 51–56. [Google Scholar] [CrossRef]
- Sim, J.H.; Tian, F.; Jung, S.Y.; Auh, J.H.; Chun, H.S. Multiplex polymerase assays for the detection of the zearalenone phenotype of Fusarium species in white and brown rice. Int. J. Food Microbiol. 2018, 269, 120–127. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; De Boevre, M.; Goryacheva, I.Y.; Werbrouck, S.; Guo, Y.; De Saeger, S. Immunochemical approach for zearalenone-4-glucoside determination. Talanta 2013, 106, 422–430. [Google Scholar] [CrossRef]
- Hervas, M.; Lopez, A.; Escarpa, A. Simplified calibration and analysis on screen-printed disposable platforms for electrochemical magnetic bead-based immunosensing of zearalenone in baby food samples. Biosens. Bioelectron. 2010, 25, 1755–1760. [Google Scholar] [CrossRef]
- Liu, L.; Chao, Y.; Cao, W.; Wang, Y.; Luo, C.; Pang, X.; Fan, D.; Wei, Q. A label-free amperometric immunosensor for detection of zearalenone based on trimetallic Au-core/AgPt-shell nanorattles and mesoporous carbon. Anal. Chim. Acta 2014, 847, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Edupuganti, S.R.; Edupuganti, O.P.; O’Kennedy, R. Generation of anti-zearalenone scFv and its incorporation into surface plasmon resonance-based assay for the detection of zearalenone in sorghum. Food Control 2013, 34, 668–674. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Chelyadina, D.S.; Gorbach, L.A.; Brovko, O.O.; Piletska, E.V.; Piletsky, S.A.; Sergeeva, L.M.; El’skaya, A.V. Colorimetric biomimetic sensor systems based on molecularly imprinted polymer membranes for highly-selective detection of phenol in environmental samples. Biopolym. Cell 2014, 30, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Sergeyeva, T.A.; Yarinka, D.V.; Piletska, E.V.; Linnik, R.P.; Zaporozhets, O.A.; Brovko, O.O.; Piletsky, S.A.; El’skaya, A.V. Fluorescent sensor systems based on nanostructured polymeric membranes for selective recognition of aflatoxin B1. Talanta 2017, 175, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.A.; Gorbach, L.A.; Piletska, E.V.; Piletsky, S.A.; Brovko, O.O.; Honcharova, L.A.; Lutsyk, O.D.; Sergeeva, L.M.; El’skaya, A.V. Colorimetric test-systems for creatinine detection based on composite molecularly imprinted polymer membranes. Anal. Chim. Acta 2013, 770, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.A.; Gorbach, L.A.; Slinchenko, O.A.; Goncharova, L.A.; Piletska, O.V.; Brovko, O.O.; Sergeeva, L.M.; El’ska, G.V. Towards development of colorimetric test-systems for phenols detection based on computationally-designed molecularly imprinted polymer membranes. Mater. Sci. Eng. C 2010, 30, 431–436. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Slinchenko, O.A.; Gorbach, L.A.; Matyushov, V.F.; Brovko, O.O.; Piletsky, S.A.; Sergeeva, L.M.; El’ska, G.V. Catalytic molecularly imprinted polymer membranes: Development of the biomimetic sensor for phenols detection. Anal. Chim. Acta 2010, 659, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Piletska, E.; Karim, K.; Coker, R.; Piletsky, S. Development of the custom polymeric materials specific for aflatoxin B1 and ochratoxin A for application with the ToxiQuant T1 sensor tool. J. Chrom. A 2010, 1217, 2543–2547. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Hong, J.I.; Chang, B.Y. Development of the smartphone-based colorimetry for multi-analyte sensing arrays. Lab Chip 2014, 14, 1725–1732. [Google Scholar] [CrossRef]
- Capoferri, D.; Álvarez-Diduk, R.; Del Carlo, M.; Compagnone, D.; Merkoçi, A. Electrochromic Molecular Imprinting Sensor for Visual and Smartphone-Based Detections. Anal. Chem. 2018, 90, 5850–5856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeyeva, T.; Yarynka, D.; Piletska, E.; Linnik, R.; Zaporozhets, O.; Brovko, O.; Piletsky, S.; El’skaya, A. Development of a smartphone-based biomimetic sensor for aflatoxin B1 detection using molecularly imprinted polymer membranes. Talanta 2019, 201, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Spirin, Y.L.; Lipatov, Y.S.; Magdinets, V.V.; Sergeeva, L.M.; Kercha, Y.Y.; Savchenko, T.T.; Vilenskaya, L.N. Polymers based on polyoxypropyleneglycol, diisocyanate, and monomethacrylic ester of ethyleneglycol. Vysokomol. Soyedin. A 1968, 8, 2116–2121. [Google Scholar]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Lagana, A. Development of a multiresidue method for analysis of major Fusarium mycotoxins in corn meal using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Wulf, G. Molecular imprinting in cross-linked materials with the aid of molecular templates—A way towards artificial antibodies. Angew. Chem. Int. Ed. Engl. 1995, 34, 1812–1832. [Google Scholar] [CrossRef]
- Fang, G.; Fan, C.; Liu, H.; Pan, M.; Zhu, H.; Wang, S.D. A novel molecularly imprinted polymer on CdSe/ZnS quantum dots for highly selective optosensing of mycotoxin zearalenone in cereal samples. RSC Adv. 2014, 4, 2764–2771. [Google Scholar] [CrossRef]
- Lemke, S.L.; Grant, P.G.; Phillips, T.D. Adsorption of zearalenone by organophilic montmorillonite clay. J. Agric. Food Chem. 1998, 46, 3789–3796. [Google Scholar] [CrossRef]
- Khalili, F.; Henni, A.; East, A.L.L. pKa values of some piperazines at (298, 303, 313, and 323) K. J. Chem. Eng. Data 2009, 54, 2914–2917. [Google Scholar] [CrossRef]
- Araujo, P. Key aspects of analytical method validation and linearity evaluation. J. Chrom. B 2009, 877, 2224–2234. [Google Scholar] [CrossRef]









| Membrane; Ratio Dummy Template:Functional Monomer | Dummy Template, mg | 1-Allylpiperazine, mg | TEGDMA/OUA 85/15, mg |
|---|---|---|---|
| MIP 1:2 | 20 | 16 | 384 |
| MIP 1:4 | 20 | 32 | 368 |
| MIP 1:6 | 20 | 48 | 352 |
| Blank 1:2 | - | 16 | 384 |
| Blank 1:4 | - | 32 | 368 |
| Blank 1:6 | - | 48 | 352 |
| Membrane; Ratio Dummy Template:Functional Monomer | Dummy Template, mg | Diethylaminoethyl Methacrylate, mg | TEGDMA/OUA 85/15, mg |
|---|---|---|---|
| MIP 1:2 | 20 | 19 | 381 |
| MIP 1:4 | 20 | 38 | 362 |
| MIP 1:6 | 20 | 58 | 342 |
| Blank 1:2 | - | 19 | 381 |
| Blank 1:4 | - | 38 | 362 |
| Blank 1:6 | - | 58 | 342 |
| Membrane; Ratio Dummy Template:Functional Monomer | Dummy Template, mg | Hydroxyethyl Methacrylate, mg | TEGDMA/OUA 85/15, mg |
|---|---|---|---|
| MIP 1:2 | 20 | 16 | 384 |
| MIP 1:4 | 20 | 32 | 368 |
| MIP 1:6 | 20 | 49 | 351 |
| Blank 1:2 | - | 16 | 384 |
| Blank 1:4 | - | 32 | 368 |
| Blank 1:6 | - | 49 | 351 |
| Membrane; Ratio Dummy Template:Functional Monomer | Dummy Template, mg | 4-Vinylpyridine, mg | TEGDMA/OUA 85/15, mg |
|---|---|---|---|
| MIP 1:2 | 20 | 13 | 387 |
| MIP 1:4 | 20 | 26 | 374 |
| MIP 1:6 | 20 | 39 | 361 |
| Blank 1:2 | - | 13 | 387 |
| Blank 1:4 | - | 26 | 374 |
| Blank 1:6 | - | 39 | 361 |
| Sample No. | Amount of Zearalenone in the Sample | Amount of Zearalenone in the Sample Determined by the MIP-Based Sensor Method |
|---|---|---|
| No. 1 maize flour “Dobrodiya Foods”, Kyiv, Ukraine | 1 µg mL−1 | 1.9 ± 0.4 µg mL−1 |
| No. 2 wheat flour “Kyivmlyn”, Kyiv, Ukraine | 3 µg mL−1 | 4 ± 0.5 µg mL−1 |
| No. 3 rye flour “Dobrodiya Foods”, Kyiv, Ukraine | 5 µg mL−1 | 5 ± 0.5 µg mL−1 |
| No. 4 Romer Labs-Check-Sample-Survey CSSMY012-M17161DZ | 114 µg kg−1 | 113.2 ± 7.8 µg kg−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeyeva, T.; Yarynka, D.; Dubey, L.; Dubey, I.; Piletska, E.; Linnik, R.; Antonyuk, M.; Ternovska, T.; Brovko, O.; Piletsky, S.; et al. Sensor Based on Molecularly Imprinted Polymer Membranes and Smartphone for Detection of Fusarium Contamination in Cereals. Sensors 2020, 20, 4304. https://0-doi-org.brum.beds.ac.uk/10.3390/s20154304
Sergeyeva T, Yarynka D, Dubey L, Dubey I, Piletska E, Linnik R, Antonyuk M, Ternovska T, Brovko O, Piletsky S, et al. Sensor Based on Molecularly Imprinted Polymer Membranes and Smartphone for Detection of Fusarium Contamination in Cereals. Sensors. 2020; 20(15):4304. https://0-doi-org.brum.beds.ac.uk/10.3390/s20154304
Chicago/Turabian StyleSergeyeva, Tetyana, Daria Yarynka, Larysa Dubey, Igor Dubey, Elena Piletska, Rostyslav Linnik, Maksym Antonyuk, Tamara Ternovska, Oleksandr Brovko, Sergey Piletsky, and et al. 2020. "Sensor Based on Molecularly Imprinted Polymer Membranes and Smartphone for Detection of Fusarium Contamination in Cereals" Sensors 20, no. 15: 4304. https://0-doi-org.brum.beds.ac.uk/10.3390/s20154304