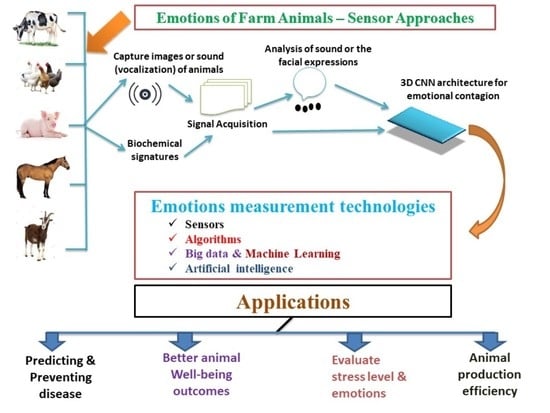
Measuring Farm Animal Emotions—Sensor-Based Approaches
Abstract
:1. Introduction
2. Animal Emotions
2.1. Emotions and Animal Welfare
2.2. Common Emotional States in Animals and Their Presentation
2.2.1. Pain
2.2.2. Fear and Aggression
2.2.3. Distress
2.3. Physiological Indicators of Emotions in Animals
2.4. Reading Animal Emotions
2.5. Relationship between Emotions, Facial Patterns, and Sounds
3. Technologies for Measuring Animal Emotions
3.1. Sensors
3.2. Global Positioning System
3.3. Thermal Infrared Imaging Sensors
3.4. Electrocardiography
3.5. Heart Rate Variability
3.6. Electroencephalography
3.7. Electromyogram
3.8. Respiratory Rate Analysis
3.9. Olfactory and Chemical Sensors
3.10. Sound Analysis Sensing Platform
4. Emotional Facial Expression
Facial Expression of Pain
5. Algorithms for Biometric Data Processing
5.1. Neural Networks
5.2. Fuzzy Logic
5.3. Support Vector Machine Algorithms
5.4. Adoption of Algorithms from Human Biometrics
6. Sensor Integration
6.1. Sound Analysis and Facial Expression
6.2. RR, HRV, and Thermal Infrared Imaging
6.3. GPS Tracking Plus Facial Expression and Recognition
6.4. Facial Expression Using Drones
7. Future Perspectives
Challenges and Opportunities
8. Research Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S.R.; Prichard, A.P.; Endres, E.L.; Newhouse, S.A.; Peters, T.L.; Crump, P.M.; Akins, M.S.; Crenshaw, T.D.; Bruckmaier, R.M.; Hernandez, L.L. Elevation of circulating serotonin improves calcium dynamics in the peripartum dairy cow. J. Endocrinol. 2016, 230, 105–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, L.E.; Veenhoven, R.; Harfeld, J.L.; Jensen, M.B. What is animal happiness? Ann. N. Y. Acad. Sci. 2019, 1438, 62–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukan, A.; Masip-Bruin, X.; Amla, N. Smart computing and sensing technologies for animal welfare: A systematic review. Comput. Soc. 2020, 50, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Fogarty, E.S.; Swain, D.L.; Cronin, G.; Trotter, M. Autonomous on-animal sensors in sheep research: A systematic review. Comput. Electron. Agric. 2018, 150, 245–256. [Google Scholar] [CrossRef]
- Kremer, L.; Holkenborg, S.E.J.K.; Reimert, I.; Bolhuis, J.E.; Webb, L.E. The nuts and bolts of animal emotion. Neurosci. Biobehav. Rev. 2020, 113, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Bekoff, M. Animal emotions: Exploring passionate natures. Bioscience 2020, 50, 861–870. [Google Scholar] [CrossRef]
- Mauss, I.B.; Robinson, M.D. Measures of emotion: A review. Cogn. Emot. 2009, 23, 209–237. [Google Scholar] [CrossRef]
- Adolphs, R. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc. Cogn. Affect. Neurosci. 2017, 12, 24–31. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.J.; Adolphs, R. A framework for studying emotions across species. Cell 2014, 157, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Adolphs, R.; Mlodinow, L.; Barrett, L.F. What is an emotion? Curr. Biol. 2019, 29, R1–R5. [Google Scholar] [CrossRef]
- Storbeck, J.; Clore, G.L. On the interdependence of cognition and emotion. Cogn. Emot. 2007, 21, 1212–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; Watkins, E.R.; Harmon-Jones, E. (Eds.) Cognition and emotion: An introduction. In Handbook of Cognition and Emotion; The Guilford Press: New York, NY, USA, 2013; pp. 3–16. ISBN 9781462509997. [Google Scholar]
- Steptoe, A.; Wardle, J.; Marmot, M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc. Natl. Acad. Sci. USA 2005, 102, 6508–6512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, D. Understanding animal welfare. Acta Vet. Scand. 2008, 50, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Jangbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2017, 92, 375–397. [Google Scholar] [CrossRef]
- Spinka, M. Social dimension of emotions and its implication for animal welfare. Appl. Anim. Behav. Sci. 2012, 138, 170–181. [Google Scholar] [CrossRef]
- Burghardt, T.; Campbell, N. December. Individual animal identification using visual biometrics on deformable coat patterns. ICVS 2007. [Google Scholar] [CrossRef]
- Nawroth, C.; Langbein, J.; Coulon, M.; Gabor, V.; Oesterwind, S.; Benz-Schwarzburg, J.; von Borell, E. Farm animal cognition—linking behavior, welfare and ethics. Front. Vet. Sci. 2019, 6, 24. [Google Scholar] [CrossRef] [Green Version]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Olsson, I.A.; Nicol, C.J.; Niemi, S.M.; Sandøe, P. From Unpleasant to Unbearable—Why and How to Implement an Upper Limit to Pain and Other Forms of Suffering in Research with Animals. Inst. Lab. Anim. Res. J. 2020. [Google Scholar] [CrossRef]
- Lischinsky, J.E.; Lin, D. Neural mechanisms of aggression across species. Nat. Neurosci. 2020, 23, 1317–1328. [Google Scholar] [CrossRef]
- Canozzi, M.E.A.; Mederos, A.; Manteca, X.; Turner, S.; McManus, C.; Zago, D.; Barcellos, J.O.J. A meta-analysis of cortisol concentration, vocalization, and average daily gain associated with castration in beef cattle. Res. Vet. Sci. 2017, 114, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef]
- Lu, Y.; Mahmoud, M.; Robinson, P. Estimating sheep pain level using facial action unit detection. In Proceedings of the 12th IEEE International Conference on Automatic Face & Gesture Recognition, Washington, DC, USA, 30 May–3 June 2017; pp. 394–399. [Google Scholar]
- McLennan, K.M.; Miller, A.L.; Dalla Costa, E.; Stucke, D.; Corke, M.J.; Broom, D.M.; Leach, M.C. Conceptual and methodological issues relating to pain assessment in mammals: The development and utilization of pain facial expression scales. Appl. Anim. Behav. Sci. 2019, 217, 1–15. [Google Scholar] [CrossRef]
- Guesgen, M.J.; Beausoleil, N.J.; Leach, M.; Minot, E.O.; Stewart, M.; Stafford, K.J. Coding and quantification of a facial expression for pain in lambs. Behav. Process. 2016, 132, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellegarde, L.G.; Haskell, M.J.; Duvaux-Ponter, C.; Weiss, A.; Boissy, A.; Erhard, H.W. Face-based perception of emotions in dairy goats. Appl. Anim. Behav. Sci. 2017, 193, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Valenchon, M.; Lévy, F.; Moussu, C.; Lansade, L. Stress affects instrumental learning based on positive or negative reinforcement in interaction with personality in domestic horses. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hintze, S.; Smith, S.; Patt, A.; Bachmann, I.; Würbel, H. Are eyes a mirror of the soul? What eye wrinkles reveal about a horse’s emotional state. PLoS ONE 2016, 11, e0164017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, R.O.; Hawthorne, S.J.; McBride, S.D. Blink rate as a measure of stress and attention in the domestic horse (Equus caballus). Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Lundblad, J. Changes in Facial Expressions During Short Term Emotional Stress as Described by a Facial Action Coding System in Horses; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018. [Google Scholar]
- Lambert, H.S.; Carder, G. Looking into the eyes of a cow: Can eye whites be used as a measure of emotional state? Appl. Anim. Behav. Sci. 2017, 186, 1–6. [Google Scholar] [CrossRef]
- Battini, M.; Agostini, A.; Mattiello, S. Understanding cows’ emotions on farm: Are eye white and ear posture reliable indicators? Animals 2019, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Proctor, H.S.; Carder, G. Nasal temperatures in dairy cows are influenced by positive emotional state. Physiol. Behav. 2015, 138, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Lidfors, L.M.; Lomax, S.; Favaro, L.; Clark, C.E. Vocal production in postpartum dairy cows: Temporal organization and association with maternal and stress behaviors. J. Dairy Sci. 2021, 104, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Gómez, Y.; Bieler, R.; Hankele, A.K.; Zähner, M.; Savary, P.; Hillmann, E. Evaluation of visible eye white and maximum eye temperature as non-invasive indicators of stress in dairy cows. Appl. Anim. Behav. Sci. 2018, 198, 1–8. [Google Scholar] [CrossRef]
- Lv, J.; Li, J.; Wang, C.; Zhao, P.; Bi, Y.; Zhang, X.; Yi, R.; Li, X.; Bao, J. Positive or negative emotion induced by feeding success or failure can affect behaviors, heart rate and immunity of suckling calves. Physiol. Behav. 2018, 196, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Rozempolska-Rucihska, I.; Czech, A.; Kasperek, K.; Zieba, G.; Ziemiahska, A. Behaviour and stress in three breeds of laying hens kept in the same environment. S. Afr. J. Anim. Sci. 2020, 50, 272–280. [Google Scholar] [CrossRef]
- Kozak, A.; Rozempolska-Rucińska, I.; Kasperek, K.; Bownik, A. Level of stress in relation to emotional reactivity of hens. Ital. J. Anim. Sci. 2019, 18, 1252–1258. [Google Scholar] [CrossRef] [Green Version]
- Marino, L.; Merskin, D. Intelligence, complexity, and individuality in sheep. Anim. Sentien. 2019, 4, 1–26. [Google Scholar] [CrossRef]
- Herborn, K.A.; McElligott, A.G.; Mitchell, M.A.; Sandilands, V.; Bradshaw, B.; Asher, L. Spectral entropy of early-life distress calls as an iceberg indicator of chicken welfare. J. R. Soc. Interface 2020, 17, 20200086. [Google Scholar] [CrossRef]
- Rius, M.M.; Pageat, P.; Bienboire-Frosini, C.; Teruel, E.; Monneret, P.; Leclercq, J.; Lafont-Lecuelle, C.; Cozzi, A. Tail and ear movements as possible indicators of emotions in pigs. Appl. Anim. Behav. Sci. 2018, 205, 14–18. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Indicators of positive and negative emotions and emotional contagion in pigs. Physiol. Behav. 2013, 109, 42–50. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Emotions on the loose: Emotional contagion and the role of oxytocin in pigs. Anim. Cogn. 2015, 18, 517–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camerlink, I.; Coulange, E.; Farish, M.; Baxter, E.M.; Turner, S.P. Facial expression as a potential measure of both intent and emotion. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Paoli, M.A.; Lahrmann, H.P.; Jensen, T.; D’Eath, R.B. Behavioural differences between weaner pigs with intact and docked tails. Anim. Welf. 2016, 25, 287–296. [Google Scholar] [CrossRef]
- Czycholl, I.; Hauschild, E.; Büttner, K.; Krugmann, K.; Burfeind, O.; Krieter, J. Tail and ear postures of growing pigs in two different housing conditions. Behav. Process. 2020, 104138. [Google Scholar] [CrossRef] [PubMed]
- Grosz, H.J.; Farmer, B.B. Blood lactate in the development of anxiety symptoms: A critical examination of Pitts and McClure’s hypothesis and experimental study. Arch. Gen. Psychiatry 1969, 21, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, C.; Altimiras, J. Point-of-care devices for physiological measurements in field conditions. A smorgasbord of instruments and validation procedures. Comp. Biochem. Phys. A 2016, 202, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Tarantola, M.; Biasato, I.; Biasibetti, E.; Biagini, D.; Capra, P.; Guarda, F.; Leporati, M.; Malfatto, V.; Cavallarin, L.; Miniscalco, B.; et al. Beef cattle welfare assessment: Use of resource and animal-based indicators, blood parameters and hair 20β-dihydrocortisol. Ital. J. Anim. Sci. 2020, 19, 341–350. [Google Scholar] [CrossRef]
- Otten, W.; Heimbürge, S.; Kanitz, E.; Tuchscherer, A. It’s getting hairy–External contamination may affect the validity of hair cortisol as an indicator of stress in pigs and cattle. Gen. Comp. Endocrinol. 2002, 113531. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J.L. Salivary oxytocin in pigs, cattle, and goats during positive human-animal interactions. Psychoneuroendocrinology 2020, 115, 104636. [Google Scholar] [CrossRef]
- López-Arjona, M.; Mateo, S.V.; Manteca, X.; Escribano, D.; Cerón, J.J.; Martínez-Subiela, S. Oxytocin in saliva of pigs: An assay for its measurement and changes after farrowing. Domest. Anim. Endocrinol. 2020, 70, 106384. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in animal cognition and behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loudon, K.M.; Tarr, G.; Pethick, D.W.; Lean, I.J.; Polkinghorne, R.; Mason, M.; Dunshea, F.R.; Gardner, G.E.; McGilchrist, P. The use of biochemical measurements to identify pre-slaughter stress in pasture finished beef cattle. Animals 2019, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzedzickis, A.; Kaklauskas, A.; Bucinskas, V. Human emotion recognition: Review of sensors and methods. Sensors 2020, 20, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briefer, E.F. Vocal contagion of emotions in non-human animals. Proc. R. Soc. B 2018, 285, 20172783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adriaense, J.E.C.; Koski, S.E.; Huber, L.; Lamm, C. Challenges in the comparative study of empathy and related phenomena in animals. Neurosci. Biobehav. Rev. 2020, 112, 62–82. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press: New York, NY, USA, 2004; ISBN 9780198025672. [Google Scholar]
- Tomkins, S.S.; McCarter, R. What and where are the primary affects? Some evidence for a theory. Percept. Mot. Skills 1964, 18, 119–158. [Google Scholar] [CrossRef]
- Di Giminiani, P.; Brierley, V.L.; Scollo, A.; Gottardo, F.; Malcolm, E.M.; Edwards, S.A.; Leach, M.C. The assessment of facial expressions in piglets undergoing tail docking and castration: Toward the development of the piglet grimace scale. Front. Vet. Sci. 2016, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lansade, L.; Nowak, R.; Lainé, A.L.; Leterrier, C.; Bonneau, C.; Parias, C.; Bertin, A. Facial expression and oxytocin as possible markers of positive emotions in horses. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Stomp, M.; Leroux, M.; Cellier, M.; Henry, S.; Lemasson, A.; Hausberger, M. An unexpected acoustic indicator of positive emotions in horses. PLoS ONE 2018, 13, e0197898. [Google Scholar] [CrossRef] [Green Version]
- Friel, M.; Kunc, H.P.; Griffin, K.; Asher, L.; Collins, L.M. Positive and negative contexts predict duration of pig vocalizations. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Lao, F.; Teng, G. A sound source localization analytical method for monitoring the abnormal night vocalizations of poultry. Sensors 2018, 18, 2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neethirajan, S. Recent advances in wearable sensors for animal health management. Sens. Bio Sens. Res. 2017, 12, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Massawe, E.A.; Michael, K.; Kaijage, S.; Seshaiyer, P. Design and Analysis of smart sensing system for animal emotions recognition. Int. J. Comput. Appl. 2017, 169, 975–8887. [Google Scholar] [CrossRef]
- Bailey, D.W.; Trotter, M.G.; Knight, C.W.; Thomas, M.G. Use of GPS tracking collars and accelerometers for rangeland livestock production research. Trans. Anim. Sci. 2018, 2, 81–88. [Google Scholar] [CrossRef]
- Neethirajan, S. Transforming the adaptation physiology of farm animals through Sensors. Animals 2020, 10, 1512. [Google Scholar] [CrossRef]
- Jeelani, R.; Jeelani, R. Thermal imagery for monitoring livestock. Int. J. Life Sci. Appl. Sci. 2019, 1, 58–69. [Google Scholar]
- Bartolomé, E.; Azcona, F.; Cañete-Aranda, M.; Perdomo-González, D.I.; Ribes-Pons, J.; Terán, E.M. Testing eye temperature assessed with infrared thermography to evaluate stress in meat goats raised in a semi-intensive farming system: A pilot study. Arch. Anim. Breed. 2019, 62, 199–204. [Google Scholar] [CrossRef]
- Cannas, S.; Palestrini, C.; Canali, E.; Cozzi, B.; Ferri, N.; Heinzl, E.; Minero, M.; Chincarini, M.; Vignola, G.; Dalla Costa, E. Thermography as a non-invasive measure of stress and fear of humans in sheep. Animals 2018, 8, 146. [Google Scholar] [CrossRef] [Green Version]
- Balters, S.; Steinert, M. Capturing emotion reactivity through physiology measurement as a foundation for affective engineering in engineering design science and engineering practices. J. Intell. Manuf. 2012, 28, 1585–1607. [Google Scholar] [CrossRef] [Green Version]
- Frondelius, L.; Järvenranta, K.; Koponen, T.; Mononen, J. The effects of body posture and temperament on heart rate variability in dairy cows. Physiol. Behav. 2015, 139, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Hayano, J. Introduction to heart rate variability. In Clinical Assessment of the Autonomic Nervous System; Iwase, S., Hayano, J., Orimo, S., Eds.; Springer: Tokyo, Japan, 2017; pp. 109–127. ISBN 978-4-431-56010-4. [Google Scholar]
- Düpjan, S.; Krause, A.; Moscovice, L.R.; Nawroth, C. Emotional contagion and its implications for animal welfare. CAB Rev. 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Goursot, C.; Düpjan, S.; Tuchscherer, A.; Puppe, B.; Leliveld, L.M. Visual laterality in pigs: Monocular viewing influences emotional reactions in pigs. Animal Behav. 2019, 154, 183–192. [Google Scholar] [CrossRef]
- Aoki, T.; Itoh, M.; Chiba, A.; Kuwahara, M.; Nogami, H.; Ishizaki, H.; Yayou, K.I. Heart rate variability in dairy cows with postpartum fever during night phase. PLoS ONE 2020, 15, e0242856. [Google Scholar] [CrossRef]
- Byrd, C.J.; Johnson, J.S.; Radcliffe, J.S.; Craig, B.A.; Eicher, S.D.; Lay, D.C. Nonlinear analysis of heart rate variability for evaluating the growing pig stress response to an acute heat episode. Animal 2020, 14, 379–387. [Google Scholar] [CrossRef]
- Lenoir, D.; Willaert, W.; Coppieters, I.; Malfliet, A.; Ickmans, K.; Nijs, J.; Vonck, K.; Meeus, M.; Cagnie, B. Electroencephalography during Nociceptive stimulation in chronic pain patients: A systematic review. Pain Med. 2020. [Google Scholar] [CrossRef]
- De Camp, N.V.; Ladwig-Wiegard, M.; Geitner, C.I.; Bergeler, J.; Thöne-Reineke, C. EEG based assessment of stress in horses: A pilot study. Peer J. 2020, 8, e8629. [Google Scholar] [CrossRef]
- Kells, N.J.; Beausoleil, N.J.; Sutherland, M.A.; Johnson, C.B. Post-natal development of EEG responses to noxious stimulation in pigs (Sus scrofa) aged 1–15 days. Anim. Welf. 2019, 28, 317–329. [Google Scholar] [CrossRef]
- Egger, M.; Ley, M.; Hanke, S. Emotion recognition from physiological signal analysis: A review. Electron. Notes Theor. Comput. Sci. 2019, 343, 35–55. [Google Scholar] [CrossRef]
- Leshin, J.C.; Lindquist, K.A. Neuroimaging of Emotion. In Oxford Handbook of Emotion Dysregulation; Oxford Handbooks: Oxford, UK, 2020. [Google Scholar]
- Klemm, W.R. Are there EEG correlates of mental states in animals? Neuropsychobiology 1992, 26, 151–165. [Google Scholar] [CrossRef]
- Leskošek, B.; Pajntar, M. Electromyography of the pregnant uterus in humans and sheep. In Proceedings of the 2nd European Medical & Biological Engineering Conference, Portorož, Slovenia, 29 November–3 December 2020; Volume 4, pp. 544–545. [Google Scholar]
- Williams, J.M. Electromyography in the horse: A useful technology. J. Equine Vet. Sci. 2018, 60, 43–58. [Google Scholar] [CrossRef]
- Valentin, S.; Zsoldos, R.R. Surface electromyography in animal biomechanics: A systematic review. J. Electromyogr. Kinesiol. 2016, 28, 167–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Chen, X.; Zhan, Q.; Yang, T.; Xia, S. Respiration-based emotion recognition with deep learning. Comput. Ind. 2017, 92, 84–90. [Google Scholar] [CrossRef]
- Lopedote, M.; Valentini, S.; Musella, V.; Vilar, J.M.; Spinella, G. Pulse rate, respiratory rate and rectal temperature in working dogs before and after three different field trials. Animals 2020, 10, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, S.; Hoffmann, G.; Ammon, C.; Heuwieser, W.; Levit, H.; Halachmi, I.; Amon, T. Effect of two cooling frequencies on respiration rate in lactating dairy cows under hot and humid climate conditions. Ann. Anim. Sci. 2019, 19, 821–834. [Google Scholar] [CrossRef] [Green Version]
- Clouard, C.M.; Bolhuis, J.E. Olfactory behaviour in farm animals. In Olfaction in Animal Behaviour and Welfare; Nielsen, B.L., Ed.; CABI Publishing: Jouy en Josas, France, 2017; pp. 161–175. ISBN 9781786391599. [Google Scholar]
- Sullivan, R.M.; Wilson, D.A.; Ravel, N.; Mouly, A.M. Olfactory memory networks: From emotional learning to social behaviors. Front. Behav. Neurosci. 2015, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Brunjes, P.C.; Feldman, S.; Osterberg, S.K. The pig olfactory brain: A primer. Chem. Senses 2016, 41, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Cramp, A.P.; Sohn, J.H.; James, P.J. Detection of cutaneous myiasis in sheep using an ‘electronic nose’. Vet. Parasitol. 2009, 166, 293–298. [Google Scholar] [CrossRef]
- Bombail, V. The Role of Olfaction in Relation to Stress and Fear. In Olfaction in Animal Behaviour and Welfare; CABI Publishing: Jouy en Josas, France, 2017. [Google Scholar]
- Adelman, J.S.; Estes, Z.; Cossu, M. Emotional sound symbolism: Languages rapidly signal valence via phonemes. Cognition 2018, 175, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Bishop, J.C.; Falzon, G.; Trotter, M.; Kwan, P.; Meek, P.D. Livestock vocalisation classification in farm soundscapes. Comp. Electron. Agric. 2019, 162, 531–542. [Google Scholar] [CrossRef]
- Da Silva, J.P.; de Alencar Nääs, I.; Abe, J.M.; da Silva Cordeiro, A.F. Classification of piglet (Sus Scrofa) stress conditions using vocalization pattern and applying paraconsistent logic Eτ. Comput. Electron. Agric. 2019, 166, 105020. [Google Scholar] [CrossRef]
- Chen, X.; Pan, Z.; Wang, P.; Yang, X.; Liu, P.; You, X.; Yuan, J. The integration of facial and vocal cues during emotional change perception: EEG markers. Soc. Cogn. Affect Neurosci. 2016, 11, 1152–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, T.; Van Reekum, C.M.; Oakes, T.R.; Davidson, R.J. The voice of emotion: An FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect Neurosci. 2006, 1, 242–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota-Rojas, D.; Orihuela, A.; Martínez-Burnes, J.; Gómez, J.; Mora-Medina, P.; Alavez, B.; Ramírez, L.; González-Lozano, M. Neurological modulation of facial expressions in pigs and implications for production. J. Anim. Behav. Biometeorol. 2020, 8, 232–243. [Google Scholar] [CrossRef]
- Ginovart-Panisello, G.J.; Alsina-Pagès, R.M.; Sanz, I.I.; Monjo, T.P.; Prat, M.C. Acoustic description of the soundscape of a real-life intensive farm and its impact on animal welfare: A preliminary analysis of farm sounds and bird vocalisations. Sensors 2020, 20, 4732. [Google Scholar] [CrossRef]
- Samadiani, N.; Huang, G.; Cai, B.; Luo, W.; Chi, C.H.; Xiang, Y.; He, J. A review on automatic facial expression recognition systems assisted by multimodal sensor data. Sensors 2019, 19, 1863. [Google Scholar] [CrossRef] [Green Version]
- McLennan, K.; Mahmoud, M. Development of an automated pain facial expression detection system for sheep (Ovis Aries). Animals 2019, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Merkies, K.; Ready, C.; Farkas, L.; Hodder, A. Eye Blink Rates and Eyelid Twitches as a Non-Invasive Measure of Stress in the Domestic Horse. Animals 2019, 9, 562. [Google Scholar] [CrossRef] [Green Version]
- Briefer Freymond, S.; Bardou, D.; Beuret, S.; Bachmann, I.; Zuberbühler, K.; Briefer, E.F. Elevated sensitivity to tactile stimuli in stereotypic horses. Front. Vet. Sci. 2019, 6, 162. [Google Scholar] [CrossRef] [Green Version]
- Descovich, K.A.; Wathan, J.; Leach, M.C.; Buchanan-Smith, H.M.; Flecknell, P.; Framingham, D.; Vick, S.J. Facial expression: An under-utilized tool for the assessment of welfare in mammals. ALTEX 2017, 34, 409–429. [Google Scholar] [CrossRef] [Green Version]
- Marsot, M.; Mei, J.; Shan, X.; Ye, L.; Feng, P.; Yan, X.; Li, C.; Zhao, Y. An adaptive pig face recognition approach using Convolutional Neural Networks. Comput. Electron. Agric. 2020, 173, 105386. [Google Scholar] [CrossRef]
- Hansen, M.F.; Smith, M.L.; Smith, L.N.; Salter, M.G.; Baxter, E.M.; Farish, M.; Grieve, B. Towards on-farm pig face recognition using convolutional neural networks. Comput. Ind. 2018, 98, 145–152. [Google Scholar] [CrossRef]
- Waller, B.M.; Julle-Daniere, E.; Micheletta, J. Measuring the evolution of facial ‘expression’ using multi-species FACS. Neurosci. Biobehav. Rev. 2020, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liakos, K.G.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine learning in agriculture: A review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koohestani, A.; Abdar, M.; Khosravi, A.; Nahavandi, S.; Koohestani, M. Integration of ensemble and evolutionary machine learning algorithms for monitoring diver behavior using physiological signals. IEEE Access 2019, 7, 98971–98992. [Google Scholar] [CrossRef]
- Sarwar, F.; Griffin, A.; Periasamy, P.; Portas, K.; Law, J. Detecting and counting sheep with a convolutional neural network. In Proceedings of the 15th IEEE International Conference on Advanced Video and Signal Based Surveillance (AVSS), Auckland, New Zeland, 27–30 November 2018; pp. 1–6. [Google Scholar]
- Chen, C.; Zhu, W.; Steibel, J.; Siegford, J.; Wurtz, K.; Han, J.; Norton, T. Recognition of aggressive episodes of pigs based on convolutional neural network and long short-term memory. Comput. Electron. Agric. 2020, 169, 105166. [Google Scholar] [CrossRef]
- Ter-Sarkisov, A.; Ross, R.; Kelleher, J.; Earley, B.; Keane, M. Beef cattle instance segmentation using fully convolutional neural network. arXiv 2018, arXiv:1807.01972. [Google Scholar]
- El-Nasr, M.S.; Yen, J.; Ioerger, T.R. Flame—fuzzy logic adaptive model of emotions. Auton. Agents Multi Agent Syst. 2000, 3, 219–257. [Google Scholar] [CrossRef]
- Borges, G.; Gates, R.S.; Sales, G.T.; da Silva Miranda, K.O. Fuzzy logic application on the determination of noise levels as an indicative of swine welfare in controlled environments. Agric. Biol. Eng. 2010, 5, 5242–5250. [Google Scholar] [CrossRef]
- Hokkanen, A.H.; Hänninen, L.; Tiusanen, J.; Pastell, M. Predicting sleep and lying time of calves with a support vector machine classifier using accelerometer data. Appl. Anim. Behav. Sci. 2011, 134, 10–15. [Google Scholar] [CrossRef]
- Boz, H.; Kose, U. Emotion extraction from facial expressions by using artificial intelligence techniques. Brain 2018, 9, 5–16. [Google Scholar]
- Munoz, R.; Olivares, R.; Taramasco, C.; Villarroel, R.; Soto, R.; Barcelos, T.S.; Merino, E.; Alonso-Sánchez, M.F. Using black hole algorithm to improve eeg-based emotion recognition. Comput. Intell. Neurosci. 2018, 3050214, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Padial, E.; Ibáñez-Molina, A.J. Fractal dimension of EEG signals and heart dynamics in discrete emotional states. Biol. Psychol. 2018, 137, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, C.; He, M.; Wang, J.; Ji, Q. Posed and spontaneous expression recognition through modeling their spatial patterns. Mach. Vis. Appl. 2015, 26, 219–231. [Google Scholar] [CrossRef]
- Nakisa, B.; Rastgoo, M.N.; Tjondronegoro, D.; Chandran, V. Evolutionary computation algorithms for feature selection of EEG-based emotional expression recognition using mobile sensors. Expert Syst. Appl. 2018, 93, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Zhi, H.; Liu, S. Face recognition based on genetic algorithm. J. Vis. Commun. Image Represent. 2019, 58, 495–502. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.; Huang, T.; Gao, W. Speech emotion recognition using deep convolutional neural network and discriminant temporal pyramid matching. IEEE Trans. Multimed. 2017, 20, 1576–1590. [Google Scholar] [CrossRef]
- Debauche, O.; Said, M.; Herinaina, A.L.; Manneback, P.; Bindelle, J.; Lebeau, F. Cloud services integration for farm animals’ behavior studies based on smartphones as activity sensors. J. Amb. Intel. Hum. Comp. 2018, 10, 4651–4662. [Google Scholar] [CrossRef] [Green Version]
- Ley, M.; Egger, M.; Hanke, S. Evaluating methods for emotion recognition based on facial and vocal features. In Proceedings of the European Conference on Ambient Intelligence, Rome, Italy, 13–15 November 2019; pp. 84–93. [Google Scholar]


| Farm Animal Species | Indicators Inferring Emotions and the Emotions/Affective States | References |
|---|---|---|
| Sheep | Horizontal ear posture—Neutral state Ears backward—Fear Ears up—Anger Asymmetric ears—Surprise | [15] |
| Sheep | Ear flat—Pain not present Ear flipped—Pain fully present/Negative state Shallow U-shaped nose—Pain not present/Neutral or positive state Extended V shaped nose—Pain fully present/Negative state Eye fully open, Pain fully present/Negative state Eye partly closed, Pain not present, Neutral or positive state | [24,25] |
| Lamb | Cheek flattening—Less bulging of nose and cheek area—Pain Ear Posture—Ears tense and point backwards or downwards (no visible inner ear)—Pain Ears relaxed and horizontal or inner ear visible—Not in pain or neutral state Flat and tight lip like horizontal line—‘Smile’ emotion and not in pain Tight nose with decreased nostril size—Pain V shape nose—in pain—U shape nose—Not in pain Squeezing or closing of eye (Orbital tightening)—In Pain | [26] |
| Goat | Ears lowered and turn down—Positive emotions Ear tips pointing backwards, and auricles turned down—Negative emotions | [27] |
| Horse | Lower oxytocin level—Neutral and positive emotional states Rise in cortisol levels and rise in heart rate parameters—Stress | [28] |
| Horse | More eye white region—Negative emotion experience Decrease in eye wrinkle expression—Positive emotion condition Increase in eye wrinkle expression—Increase in negative emotion | [29] |
| Horse | Increase in spontaneous blink rate of eye—Stress Increase in dopamine levels—Positive emotion due to reward Increase in salivary cortisol and change in heart rate variability—Stress | [30] |
| Horse | Increase in heart rate, eye white increase, nostril dilator, upper eyelid raiser, inner brow raiser, tongue show with increase in ear flicker and blink frequency—All related to increase in stress | [31] |
| Cow | Upright ear posture longer—Excitement Forward facing ear posture—Frustration | [32] |
| Cow | Half-closed eyes and ears backwards or hung-down—Relaxed State Eye white clearly visible and ears directed forward—Excited State | [33] |
| Cow | Decrease in nasal temperature and change in peripheral temperature—Positive experience or increase in arousal | [34] |
| Cow | Cow vocalizations—Open mouth calls & a greater number of vocal units per sequence—alert and stress escalation Close mouth calls—Positive emotional state | [35] |
| Cow | Visible eye white and maximum eye temperature—Stress | [36] |
| Dairy Calves | Lower heart rate—positive emotion Higher heart rate—negative emotion Increase in salivary cortisol—Both positive and negative emotion Higher secretory immunoglobulin A (SIgA), Serum IL-2 and IL-3 levels—Positive emotional states Higher serum of tumor necrosis factor alpha (TNFα)—Negative emotion | [37] |
| Hens | Increase in cortisol in serum—Negative emotions and stress Increase in corticosterone levels in feathers—Positive emotions | [38] |
| Hens | Increase in corticosterone levels in feathers—Positive excited states | [39] |
| Chickens | Tachycardia and bodily fever—Fear Increased locomotion and pacing behaviour—Anxiety or Negative emotion Lower corticosterone—Positive emotion | [40] |
| Chickens | Repetitive, high energy calls (sounds)—Distress or negative emotions | [41] |
| Pigs | High frequency ear movement—Stress or negative emotion High duration lateral tail movement—Positive emotions or play behavior | [42] |
| Pigs | Tail raised and forming a loop—Positive emotion Ears forward—Alert and neutral emotion Ears backward—Negative emotion Hanging ears flipping in the direction of eyes—Normal state (Neutral emotion Standing upright ears—Normal neutral state | [43,44] |
| Pigs | Smaller snout ration and ears forward oriented—Aggression or negative emotion state Ears backward and less open eyes—Retreat from aggression or transition to neutral state | [45] |
| Pigs | Tail hang loose—Negative or neutral emotion state | [46] |
| Pigs | Curled up tails and ears directed forward—Positive emotion state Tucked under tails—Negative emotion | [47] |
| System | Pros | Cons |
|---|---|---|
| Global Positioning System | Long-lasting system, noninvasive | Expensive at startup, battery life, issues with accuracy, noise |
| Thermal Infrared Imaging | Accurate indicator of temperature, noninvasive | Subject to interference from external heat sources |
| Electrocardiograph | Likely reliable indicator of positive affect through heart rate measurement | Deployability issues due to motion artefacts; Not practical for real-time or on-site monitoring |
| Electroencephalography | Accurate measure of brain activity irrespective of subject movement | Dissociation between EEG states and emotional valences; Real-time non-invasive sensors are not yet available |
| Electromyogram | Useful for many diagnostics | Subject to interference; Only measures surface muscles |
| Respirometer | Especially useful for diagnostics and for animals with distinct breath patterns | Difficult to implement and influenced by many factors including motion |
| Olfactory and chemical sensors | Strongly linked to emotion | Do not use data from the animal directly; Indirect measurement as validated benchmarks is unavailable |
| System | Features |
|---|---|
| Neural Networks | Employs many distinct algorithms together; Can mimic the brain of the target species |
| Fuzzy Logic | Compares continuous inputs to known baselines |
| Support Vector Machine Algorithms | Useful in classification; good for establishing broad relationships between many variables |
| Company | Project |
|---|---|
| Iris Data Science, New Zealand | Prototyped sheep reidentification system designed to be affordable for farmers |
| Moofarm, India | Cattle reidentification system |
| SmartAHC, Singapore | Livestock management technologies for record-keeping, facial recognition, health management, and quality assurance |
| Yingzi Technology, China | Facial recognition system for pigs used for tracking, preventing meat fraud, and working towards productivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neethirajan, S.; Reimert, I.; Kemp, B. Measuring Farm Animal Emotions—Sensor-Based Approaches. Sensors 2021, 21, 553. https://0-doi-org.brum.beds.ac.uk/10.3390/s21020553
Neethirajan S, Reimert I, Kemp B. Measuring Farm Animal Emotions—Sensor-Based Approaches. Sensors. 2021; 21(2):553. https://0-doi-org.brum.beds.ac.uk/10.3390/s21020553
Chicago/Turabian StyleNeethirajan, Suresh, Inonge Reimert, and Bas Kemp. 2021. "Measuring Farm Animal Emotions—Sensor-Based Approaches" Sensors 21, no. 2: 553. https://0-doi-org.brum.beds.ac.uk/10.3390/s21020553