Potentiometric Performance of a Highly Flexible-Shaped Trifunctional Sensor Based on ZnO/V2O5 Microrods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
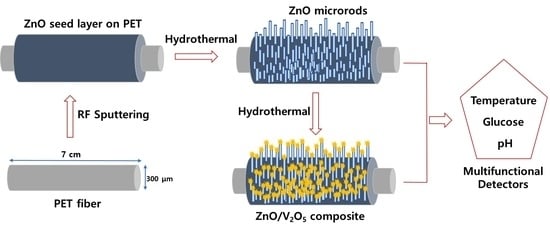
2.2. Fabrication of ZnO/V2O5 on ZnO Sputtered PET Fiber
2.2.1. ZnO Seed Deposition
2.2.2. Simultaneous Hydrothermal Growth of ZnO and ZnO/V2O5
2.3. Material Characterization
2.4. Construction and TCR Measurement of Temperature Sensor on PET Fiber
2.5. Construction of Electrochemical Sensor on PET Fiber
2.5.1. Enzymatic Glucose Sensing Analysis
2.5.2. pH Sensing Analysis
3. Results and Discussion
3.1. Materials
3.2. Temperature Sensor
3.3. Electrochemical Sensor
3.3.1. Glucose Sensor
3.3.2. pH Sensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, Y.; Thielens, A.; Muin, S.; Ting, J.; Baumbauer, C.; Arias, A.C. A New Frontier of Printed Electronics: Flexible Hybrid Electronics. Adv. Mater. 2020, 32, e1905279. [Google Scholar] [CrossRef]
- Poh, M.Z.; Swenson, N.C.; Picard, R.W. A wearable sensor for unobtrusive, long-term assessment of electrodermal activity. IEEE Trans. Biomed. Eng. 2010, 57, 1243–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.Q.; Zhu, W.B.; Yu, X.G.; Huang, P.; Fu, S.Y.; Hu, N.; Liao, K. Multifunctional Wearable Device Based on Flexible and Conductive Carbon Sponge/Polydimethylsiloxane Composite. ACS Appl. Mater. Interfaces 2016, 8, 33189–33196. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Lee, P.; Chou, J.B.; Xu, R.; Zhao, R.; Hart, A.J.; Kim, S.G. Extremely Elastic Wearable Carbon Nanotube Fiber Strain Sensor for Monitoring of Human Motion. ACS Nano 2015, 9, 5929–5936. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Zhang, Z.; Sun, P.; Chen, H. High-Sensitivity Wearable and Flexible Humidity Sensor Based on Graphene Oxide/Non-Woven Fabric for Respiration Monitoring. Langmuir 2020, 36, 9443–9448. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Peng, S.; Blanloeuil, P.; Wu, S.; Wang, C.H. Wearable Temperature Sensors with Enhanced Sensitivity by Engineering Microcrack Morphology in PEDOT:PSS-PDMS Sensors. ACS Appl. Mater. Interfaces 2020, 12, 36578–36588. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, Y.; Zhou, R.; Meng, L.; Chen, T.; Mai, W.; Pan, C. Recent advances of wearable and flexible piezoresistivity pressure sensor devices and its future prospects. J. Mater. 2020, 6, 86–101. [Google Scholar] [CrossRef]
- Parlak, O.; Curto, V.F.; Ojeda, E.; Basabe-Desmonts, L.; Benito-Lopez, F.; Salleo, A. Wearable biosensors and sample handling strategies. In Wearable Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 65–88. ISBN 9780081024072. [Google Scholar]
- Tan, C.; Dong, Z.; Li, Y.; Zhao, H.; Huang, X.; Zhou, Z.; Jiang, J.W.; Long, Y.Z.; Jiang, P.; Zhang, T.Y.; et al. A high performance wearable strain sensor with advanced thermal management for motion monitoring. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible potentiometric pH sensors for wearable systems. RSC Adv. 2020, 10, 8594–8617. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Zhu, Y. Printing Conductive Nanomaterials for Flexible and Stretchable Electronics: A Review of Materials, Processes, and Applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Yuen, J.D.; Baingane, A.; Hasan, Q.; Shriver-Lake, L.C.; Walper, S.A.; Zabetakis, D.; Breger, J.C.; Stenger, D.A.; Slaughter, G. A Fully-Flexible Solution-Processed Autonomous Glucose Indicator. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Su, W.; Li, Z.; Liu, W.; Liu, S.; Ding, X. A modularized and flexible sensor based on MWCNT/PDMS composite film for on-site electrochemical analysis. J. Electroanal. Chem. 2017, 806, 68–74. [Google Scholar] [CrossRef]
- Bi, C.; Chen, B.; Wei, H.; DeLuca, S.; Huang, J. Efficient Flexible Solar Cell based on Composition-Tailored Hybrid Perovskite. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Hilal, A.M.; Han, B.J.I. Development of a Highly Flexible and Durable Fiber-Shaped Temperature Sensor based on Graphene/Ni Double-Decked Layer for Wearable Devices. IEEE Sens. J. 2020. [Google Scholar] [CrossRef]
- Eom, T.H.; Han, J.I. The effect of the nickel and chromium concentration ratio on the temperature coefficient of the resistance of a Ni–Cr thin film-based temperature sensor. Sens. Actuators A Phys. 2017, 260, 198–205. [Google Scholar] [CrossRef]
- Kaidarova, A.; Khan, M.A.; Marengo, M.; Swanepoel, L.; Przybysz, A.; Muller, C.; Fahlman, A.; Buttner, U.; Geraldi, N.R.; Wilson, R.P.; et al. Wearable multifunctional printed graphene sensors. npj Flex. Electron. 2019, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Zhu, Y. Wearable multifunctional sensors using printed stretchable conductors made of silver nanowires. Nanoscale 2014, 6, 2345–2352. [Google Scholar] [CrossRef]
- Girija, K.G.; Somasundaram, K.; Topkar, A.; Vatsa, R.K. Highly selective H2S gas sensor based on Cu-doped ZnO nanocrystalline films deposited by RF magnetron sputtering of powder target. J. Alloys Compd. 2016, 684, 15–20. [Google Scholar] [CrossRef]
- Liao, X.; Liao, Q.; Zhang, Z.; Yan, X.; Liang, Q.; Wang, Q.; Li, M.; Zhang, Y. A Highly Stretchable ZnO@Fiber-Based Multifunctional Nanosensor for Strain/Temperature/UV Detection. Adv. Funct. Mater. 2016, 26, 3074–3081. [Google Scholar] [CrossRef]
- Hasan, S.A.; Gibson, D.; Song, S.; Wu, Q.; Ng, W.P.; McHale, G.; Dean, J.; Fu, Y.Q. ZnO thin film based flexible temperature sensor. In Proceedings of the IEEE Sensors, Glasgow, UK, 29 October–1 November 2017; pp. 1–3. [Google Scholar]
- Marie, M.; Mandal, S.; Manasreh, O. An Electrochemical Glucose Sensor Based on Zinc Oxide Nanorods. Sensors 2015, 15, 18714–18723. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Bhat, B.; Khan, G.R.; Asokan, K. Role of substrate effects on the morphological, structural, electrical and thermoelectrical properties of V2O5 thin films. RSC Adv. 2015, 5, 52602–52611. [Google Scholar] [CrossRef]
- Pan, G.X.; Xia, X.H.; Cao, F.; Chen, J.; Zhang, Y.J. Carbon cloth supported vanadium pentaoxide nanoflake arrays as high-performance cathodes for lithium ion batteries. Electrochim. Acta 2014, 149, 349–354. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Zhou, J.; Liu, X.; Chen, Z.; Cao, C.; Luo, H.; Kanehira, M. Growth of oriented vanadium pentaoxide nanostructures on transparent conducting substrates and their applications in photocatalysis. J. Solid State Chem. 2014, 214, 79–85. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Pak, Y.; Jeong, Y.; Kim, W.; Kim, J.; Jung, G.Y. Amorphous Pd-assisted H2 detection of ZnO nanorod gas sensor with enhanced sensitivity and stability. Sens. Actuators B Chem. 2018, 262, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Yan, S.; An, L.; Chen, W.; Yang, S.; Zhao, C.; Dai, Y. Enhancement of ethanol gas sensing response based on ordered V2O5 nanowire microyarns. Sens. Actuators B Chem. 2015, 206, 284–290. [Google Scholar] [CrossRef]
- Vijayakumar, Y.; Mani, G.K.; Ponnusamy, D.; Shankar, P.; Kulandaisamy, A.J.; Tsuchiya, K.; Rayappan, J.B.B.; Ramana Reddy, M.V. V2O5 nanofibers: Potential contestant for high performance xylene sensor. J. Alloys Compd. 2018, 731, 805–812. [Google Scholar] [CrossRef]
- Kesim, Y.E.; Battal, E.; Tanrikulu, M.Y.; Okyay, A.K. An all-ZnO microbolometer for infrared imaging. Infrared Phys. Technol. 2014, 67, 245–249. [Google Scholar] [CrossRef]
- Xue, F.; Zhang, L.; Tang, W.; Zhang, C.; Du, W.; Wang, Z.L. Piezotronic effect on ZnO nanowire film based temperature sensor. ACS Appl. Mater. Interfaces 2014, 6, 5955–5961. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M. D-Glucose sensor based on ZnO/V2O5 NRs by an enzyme-free electrochemical approach. RSC Adv. 2019. [Google Scholar] [CrossRef] [Green Version]
- Eom, T.H.; Han, J.I. Resistive behavior of Ni thin film on a cylindrical PET monofilament with temperature for wearable computing devices. Sens. Actuators A Phys. 2017, 259, 96–104. [Google Scholar] [CrossRef]
- Anusha, J.R.; Kim, H.J.; Fleming, A.T.; Das, S.J.; Yu, K.H.; Kim, B.C.; Raj, C.J. Simple fabrication of ZnO/Pt/chitosan electrode for enzymatic glucose biosensor. Sens. Actuators B Chem. 2014, 202, 827–833. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Zhang, X.; Cao, B.Y.; Fujii, M.; Takahashi, K.; Ikuta, T. Influence of grain boundary scattering on the electrical properties of platinum nanofilms. Appl. Phys. Lett. 2006, 89, 114102. [Google Scholar] [CrossRef]
- Wang, Y.T.; Whang, W.T.; Chen, C.H. Hollow V2O5 nanoassemblies for high-performance room-temperature hydrogen sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487. [Google Scholar] [CrossRef]
- Ahmad, R.; Tripathy, N.; Khan, M.Y.; Bhat, K.S.; Ahn, M.S.; Hahn, Y.B. Ammonium ion detection in solution using vertically grown ZnO nanorod based field-effect transistor. RSC Adv. 2016, 6, 54836–54840. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, G.; Yang, H.; Bi, W.; Liang, X.; Zhang, Y.; Zhang, G.; Wu, G. Controlled synthesis of V2O5/MWCNT core/shell hybrid aerogels through a mixed growth and self-assembly methodology for supercapacitors with high capacitance and ultralong cycle life. J. Mater. Chem. A 2015, 3, 15692–15699. [Google Scholar] [CrossRef]
- Fang, B.; Zhang, C.; Wang, G.; Wang, M.; Ji, Y. A glucose oxidase immobilization platform for glucose biosensor using ZnO hollow nanospheres. Sens. Actuators B Chem. 2011, 155, 304–310. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhang, W.D.; Ye, J.S. Nonenzymatic electrochemical glucose sensor based on MnO2/MWNTs nanocomposite. Electrochem. Commun. 2008, 10, 1268–1271. [Google Scholar] [CrossRef]
- Sun, J.; Li, C.; Qi, Y.; Guo, S.; Liang, X. Optimizing colorimetric assay based on V2O5 nanozymes for sensitive detection of H2O2 and glucose. Sensors 2016, 16, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Pan, T.; Yang, Z. Flexible polyethylene terephthalate/polyaniline composite paper with bending durability and effective electromagnetic shielding performance. Chem. Eng. J. 2020, 389, 124433. [Google Scholar] [CrossRef]
- Hussain, M.M.; Asiri, A.M.; Arshad, M.N.; Rahman, M.M. Fabrication of a Ga3+ sensor probe based on methoxybenzylidenebenzenesulfonohydrazide (MBBSH) by an electrochemical approach. New J. Chem. 2018, 42, 1169–1180. [Google Scholar] [CrossRef]
- Mani, G.K.; Morohoshi, M.; Yasoda, Y.; Yokoyama, S.; Kimura, H.; Tsuchiya, K. ZnO-Based Microfluidic pH Sensor: A Versatile Approach for Quick Recognition of Circulating Tumor Cells in Blood. ACS Appl. Mater. Interfaces 2017, 9, 5193–5203. [Google Scholar] [CrossRef] [PubMed]








| Electrode | Method of Detection | LOD 1 (µM) | LDR 2 (µM) | Sensitivity (μAmM−1cm−2) | Reference |
|---|---|---|---|---|---|
| MnO2/MWCNT | Amperometry | - | 10–28,000 | 33.19 | [42] |
| V2O5 nanoenzymes | Chronoamperometry | 10 | 10–2000 | - | [43] |
| ZnO NR | Amperometry | 0.22 | - | 0.0109 | [23] |
| ZnO/V2O5 NR | I-V | 125,250 | 1–1000 | 1.27 | [33] |
| ZnO/V2O5 on PET | Amperometry | 174 | 10–10,000 | 72.06 | This work |
| IBM | CR, uA | IE (%) | |||
|---|---|---|---|---|---|
| R1 | R2 | R3 | Avg | ||
| G | 20.32 | 18.68 | 12.64 | 17.21 | 100 |
| AA | 3.20 | 4.31 | 3.11 | 3.54 | 20.6 |
| D | 5.61 | 4.64 | 4.16 | 4.80 | 28.9 |
| UA | 1.93 | 3.12 | 2.86 | 2.64 | 15.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiagyei, A.B.; Han, J.I. Potentiometric Performance of a Highly Flexible-Shaped Trifunctional Sensor Based on ZnO/V2O5 Microrods. Sensors 2021, 21, 2559. https://0-doi-org.brum.beds.ac.uk/10.3390/s21072559
Appiagyei AB, Han JI. Potentiometric Performance of a Highly Flexible-Shaped Trifunctional Sensor Based on ZnO/V2O5 Microrods. Sensors. 2021; 21(7):2559. https://0-doi-org.brum.beds.ac.uk/10.3390/s21072559
Chicago/Turabian StyleAppiagyei, Alfred Bekoe, and Jeong In Han. 2021. "Potentiometric Performance of a Highly Flexible-Shaped Trifunctional Sensor Based on ZnO/V2O5 Microrods" Sensors 21, no. 7: 2559. https://0-doi-org.brum.beds.ac.uk/10.3390/s21072559