Degradation Study of Thin-Film Silicon Structures in a Cell Culture Medium
Abstract
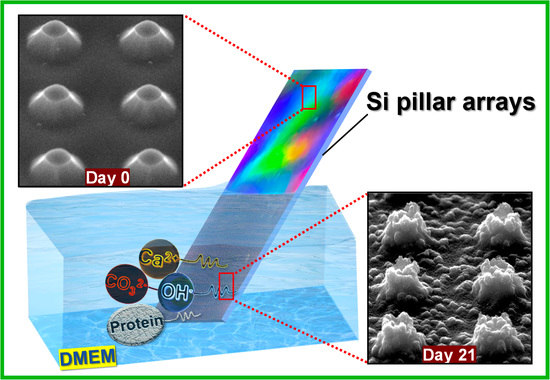
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Planar Si Thin Films and Si Pillar Arrays, and Their Degradation Experiments
2.2. In Vitro Degradation of Thin-Film Si Structures
2.3. Characterizations of Materials
2.4. Mechanical Characterization
2.5. Measurement of Electrochemical Impedance Spectra (EIS)
2.6. Cell Culture
3. Results and Discussion
3.1. Characterization of Surface Topography and Dissolution Rate
3.2. Analysis of Surface Chemistry and Physical States
3.3. Mechanical and Electrochemical Properties
3.4. In Vitro Biocompatibility Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jiang, Y.; Tian, B. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 2018, 3, 473–490. [Google Scholar] [CrossRef]
- Wang, H.C.; Sun, P.C.; Yin, L.; Sheng, X. 3D electronic and photonic structures as active biological interfaces. InfoMat 2020, 2, 527–552. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Carvalho-de-Souza, J.L.; Wong, R.C.; Luo, Z.; Isheim, D.; Zuo, X.; Nicholls, A.W.; Jung, I.W.; Yue, J.; Liu, D.J.; et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 2016, 15, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Estevez, C.; Duch, M.; Duque, M.; Del Campo, F.J.; Enriquez-Barreto, L.; Murillo, G.; Torras, N.; Plaza, J.A.; Saura, C.A.; Esteve, J. Suspended Silicon Microphotodiodes for Electrochemical and Biological Applications. Small 2017, 13, 1701920. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rogers, J.A. Interface Engineering of Si Hybrid Nanostructures for Chemical and Biological Sensing. Adv. Mater. Technol. 2020, 5, 2000380. [Google Scholar] [CrossRef]
- Ahoulou, S.; Perret, E.; Nedelec, J.-M. Functionalization and Characterization of Silicon Nanowires for Sensing Applications: A Review. Nanomaterials 2021, 11, 999. [Google Scholar] [CrossRef]
- Tintelott, M.; Pachauri, V.; Ingebrandt, S.; Vu, X.T. Process Variability in Top-Down Fabrication of Silicon Nanowire-Based Biosensor Arrays. Sensors 2021, 21, 5153. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Li, X.J.; Liu, B.; Yi, J.; Fang, Y.; Shi, F.Y.; Gao, X.; Sudzilovsky, E.; Parameswaran, R.; Koehler, K.; et al. Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng. 2018, 2, 508–521. [Google Scholar] [CrossRef]
- Parameswaran, R.; Carvalho-de-Souza, J.L.; Jiang, Y.; Burke, M.J.; Zimmerman, J.F.; Koehler, K.; Phillips, A.W.; Yi, J.; Adams, E.J.; Bezanilla, F.; et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 2018, 13, 260–266. [Google Scholar] [CrossRef]
- McCracken, J.M.; Xu, S.; Badea, A.; Jang, K.-I.; Yan, Z.; Wetzel, D.J.; Nan, K.; Lin, Q.; Han, M.; Anderson, M.A.; et al. Deterministic Integration of Biological and Soft Materials onto 3D Microscale Cellular Frameworks. Adv. Biosyst. 2017, 1, 1700068. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Fu, T.-M.; Liu, J.; Lieber, C.M. Nanoelectronics-biology frontier: From nanoscopic probes for action potential recording in live cells to three-dimensional cyborg tissues. Nano Today 2013, 8, 351–373. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Liu, J.; Dvir, T.; Jin, L.; Tsui, J.H.; Qing, Q.; Suo, Z.; Langer, R.; Kohane, D.S.; Lieber, C.M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012, 11, 986–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Yang, Z.; Meacham, K.; Cvetkovic, C.; Corbin, E.A.; Vázquez-Guardado, A.; Xue, M.; Yin, L.; Boroumand, J.; Pakeltis, G.; et al. Biodegradable Monocrystalline Silicon Photovoltaic Microcells as Power Supplies for Transient Biomedical Implants. Adv. Energy Mater. 2018, 8, 1703035. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L.; Wang, H.; Zhang, B.; Wang, X.; Stening, R.Y.Z.; Sheng, X.; Yin, L. Materials Strategies and Device Architectures of Emerging Power Supply Devices for Implantable Bioelectronics. Small 2020, 16, e1902827. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Vepachedu, V. Recent development of transient electronics. Theor. App. Mech. Lett. 2016, 6, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.W.; Park, G.; Cheng, H.; Song, J.K.; Kang, S.K.; Yin, L.; Kim, J.H.; Omenetto, F.G.; Huang, Y.; Lee, K.M.; et al. 25th anniversary article: Materials for high-performance biodegradable semiconductor devices. Adv. Mater. 2014, 26, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.K.; Emon, M.A.B.; Li, C.S.; Yang, Q.S.; Chang, H.P.; Yang, Z.J.; Wu, C.I.; Saif, M.T.; Rogers, J.A. Cytotoxicity and in Vitro Degradation Kinetics of Foundry-Compatible Semiconductor Nanomembranes and Electronic Microcomponents. ACS Nano 2018, 12, 9721–9732. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Hwang, S.-W.; Cheng, H.; Yu, S.; Kim, B.H.; Kim, J.-H.; Huang, Y.; Rogers, J.A. Dissolution Behaviors and Applications of Silicon Oxides and Nitrides in Transient Electronics. Adv. Funct. Mater. 2014, 24, 4427–4434. [Google Scholar] [CrossRef]
- Kang, S.-K.; Hwang, S.-W.; Yu, S.; Seo, J.-H.; Corbin, E.A.; Shin, J.; Wie, D.S.; Bashir, R.; Ma, Z.; Rogers, J.A. Biodegradable Thin Metal Foils and Spin-On Glass Materials for Transient Electronics. Adv. Funct. Mater. 2015, 25, 1789–1797. [Google Scholar] [CrossRef]
- Hwang, S.W.; Song, J.K.; Huang, X.; Cheng, H.; Kang, S.K.; Kim, B.H.; Kim, J.H.; Yu, S.; Huang, Y.; Rogers, J.A. High-performance biodegradable/transient electronics on biodegradable polymers. Adv. Mater. 2014, 26, 3905–3911. [Google Scholar] [CrossRef]
- Bai, W.; Shin, J.; Fu, R.; Kandela, I.; Lu, D.; Ni, X.; Park, Y.; Liu, Z.; Hang, T.; Wu, D.; et al. Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity. Nat. Biomed. Eng. 2019, 3, 644–654. [Google Scholar] [CrossRef]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; Macewan, R.M.; Huang, Y.; et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.K.; Yu, K.J.; Song, E.; Farimani, A.B.; Vitale, F.; Xie, Z.; Yoon, Y.; Kim, Y.; Richardson, A.; Luan, H.; et al. Dissolution of Monocrystalline Silicon Nanomembranes and Their Use as Encapsulation Layers and Electrical Interfaces in Water-Soluble Electronics. ACS Nano 2017, 11, 12562–12572. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Park, G.; Edwards, C.; Corbin, E.A.; Kang, S.-K.; Cheng, H.; Song, J.-K.; Kim, J.-H.; Yu, S.; Ng, J.; et al. Dissolution Chemistry and Biocompatibility of Single-Crystalline Silicon Nanomembranes and Associated Materials for Transient Electronics. ACS Nano 2014, 8, 5843–5851. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Y.; Dai, F.; Kong, D.; Wang, H.; Sun, P.; Shi, Z.; Sheng, X.; Xu, B.; Yin, L. Geometrical and Chemical-Dependent Hydrolysis Mechanisms of Silicon Nanomembranes for Biodegradable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 18013–18023. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Park, G.; Kim, K.; Hwang, S.W.; Cheng, H.; Shin, J.; Chung, S.; Kim, M.; Yin, L.; Lee, J.C.; et al. Dissolution chemistry and biocompatibility of silicon- and germanium-based semiconductors for transient electronics. ACS Appl. Mater. Interfaces 2015, 7, 9297–9305. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, H.; Cheng, D.; Ding, H.; Kong, D.; Li, L.; Yin, L.; Zhao, G.; Liu, L.; Zou, G.; et al. Diamond thin films integrated with flexible substrates and their physical, chemical and biological characteristics. J. Phys. D Appl. Phys. 2021, 54, 384004. [Google Scholar] [CrossRef]
- Yin, L.; Farimani, A.B.; Min, K.; Vishal, N.; Lam, J.; Lee, Y.K.; Aluru, N.R.; Rogers, J.A. Mechanisms for hydrolysis of silicon nanomembranes as used in bioresorbable electronics. Adv. Mater. 2015, 27, 1857–1864. [Google Scholar] [CrossRef]
- Kroemer, H. Heterostructure Devices—A Device Physicist Looks at Interfaces. Surf. Sci. 1983, 132, 543–576. [Google Scholar] [CrossRef]
- Rosenwaks, Y.; Tal, O.; Saraf, S.; Schwarzman, A.; Lepkifker, E.; Boag, A. Kelvin Probe Force Microscopy: Recent Advances and Applications. In Applied Scanning Probe Methods Viii: Scanning Probe Microscopy Techniques; Springer: Berlin/Heidelberg, Germany, 2008; pp. 351–376. [Google Scholar]
- Kozai, T.D.Y.; Catt, K.; Li, X.; Gugel, Z.V.; Olafsson, V.T.; Vazquez, A.L.; Cui, X.T. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials 2015, 37, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyaya, A.M.; Srivastava, M.C.; Sharan, P.; Roy, S.K. Silicon nanostructure-based photonic MEMS sensor for biosensing application. J. Nanophotonics 2021, 15, 026001. [Google Scholar] [CrossRef]
- Cody, P.A.; Eles, J.R.; Lagenaur, C.F.; Kozai, T.D.Y.; Cui, X.T. Unique electrophysiological and impedance signatures between encapsulation types: An analysis of biological Utah array failure and benefit of a biomimetic coating in a rat model. Biomaterials 2018, 161, 117–128. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Tian, J.; Lu, B.; Xie, Y.; Sun, P.; Yin, L.; Wang, Y.; Sheng, X. Degradation Study of Thin-Film Silicon Structures in a Cell Culture Medium. Sensors 2022, 22, 802. https://0-doi-org.brum.beds.ac.uk/10.3390/s22030802
Wang H, Tian J, Lu B, Xie Y, Sun P, Yin L, Wang Y, Sheng X. Degradation Study of Thin-Film Silicon Structures in a Cell Culture Medium. Sensors. 2022; 22(3):802. https://0-doi-org.brum.beds.ac.uk/10.3390/s22030802
Chicago/Turabian StyleWang, Huachun, Jingjing Tian, Bingwei Lu, Yang Xie, Pengcheng Sun, Lan Yin, Yuguang Wang, and Xing Sheng. 2022. "Degradation Study of Thin-Film Silicon Structures in a Cell Culture Medium" Sensors 22, no. 3: 802. https://0-doi-org.brum.beds.ac.uk/10.3390/s22030802