A Reliable Method of Measuring the Conversion Degrees of Methacrylate Dental Resins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Resins
2.2. Measuring Apparatus and Light Sources
- (a)
- Spectrophotometer IS50 ATR, FTIR/FT Raman (Thermo Scientific, Waltham, MA, USA).
- (b)
- Fluorymeter FS 900 (Edinburgh Instr., Livingston, Scotland) for measuring spectra of emission of light sources.
- (c)
- Spectrophotometer Evolution 220 (Thermo Scientific, Waltham, MA, USA) for measuring absorption spectra.
- (d)
- Power meter LaserStar Dual Channel (Ophir Laser Measurement Group, Logan, UT, USA).
2.3. Sample Preparation
2.4. Analysis
3. Results
3.1. Analysis of the Efficiency of Light Sources
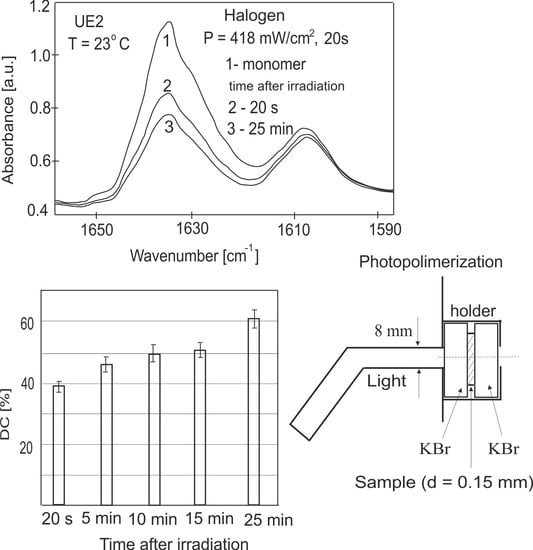
3.2. Measurements of the Conversion Degrees of the Dental Resins
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pratap, B.; Gupta, R.K.; Bhardway, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar]
- Yaclav, R.; Kumar, M. Dental restorative composite materials: A review. J. Oral Biosci. 2019, 61, 78–833. [Google Scholar]
- Ilie, N.; Obermaier, J.; Durner, J. Effect of modulated irradiation time on the degree of conversion and the amount of elutable substances from nano-hybrid resin-based composites. Clin. Oral Investig. 2014, 18, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Frauscher, K.; Ilie, N. Degree of conversion of nano-hybrid resin-based composites with novel and conventional matrix formulation. Clin. Oral Investig. 2013, 17, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, B.; Cobanoglu, N.; Cetin, A.R.; Gunduz, B. Conversion degrees of resin composites using different light sources. Eur. J. Dent. 2013, 7, 102–109. [Google Scholar] [PubMed]
- Moraes, L.G.P.; Rocha, R.S.F.; Menegazzo, L.M.; de Araujo, E.B.; Yukimito, K.; Moraes, J.C.S. Infrared spectroscopy: A tool for determination of the degree of conversion in dental composites. J. Appl. Oral Sci. 2008, 16, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Sarosi, C.; Moldovan, M.; Soanca, A.; Roman, A.; Gherman, T.; Trifoi, A.; Chisnoiu, A.M.; Cuc, S.; Filip, M.; Gheorghe, G.F.; et al. Effects of Monomer Composition of Urethane Metacrylate Based Resins on C=C Degree of Conversion, Residual Monomer Content and Mechanical Properties. Polymers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Rodrıguez-Lozano, F.J.; Serrano-Belmonte, I.; Perez Calvo, J.C.; Coronado-Parra, M.T.; Bernabeu-Esclapez, A.; Moraleda, J.M. Effects of two low-shrinkage composites on dental stem cells (viability, cell damaged or apoptosis and mesenchymal markers expression. J. Mater. Sci. Mater. Med. 2013, 24, 979–988. [Google Scholar] [CrossRef]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Urban, V.M.; Machado, A.; Vergani, C.E.; Giampaolo, E.T.; Pavarina, A.C.; Almeida, F.G.; Cass, Q.B. Effect of water-bath post-polymerization on the mechanical properties, degree of conversion, and leaching of residual compounds of hard chairside reline resins. Dent. Mater. 2009, 25, 662–671. [Google Scholar] [CrossRef]
- Ferracane, J.L.; Greener, E.H. Fourier transform infrared analysis of degree of polymerization in unfilled resins methods comparison. J. Dent. Res. 1984, 63, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Peutzfeldt, A.; Asmussen, E. The effect of posturing on the quantity of remaining double bonds, mechanical properties, and in vitro wear of two resin composites. J. Dent. 2000, 28, 447–452. [Google Scholar] [CrossRef]
- Antonucci, J.M.; Toth, E.E. Extent of polymerization of dental resins by differential scanning calorimetry. J. Dent. Res. 1983, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Sustercic, D.; Cevc, P.; Funduk, N.; Pintar, M.M. Determination of curing time in visible-cured composite resins of different thickness by electron paramagnetic resonance. J. Mater. Sci. Mater. Med. 1997, 8, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.R.; Kalachandra, S.; Shobla, H.K.; Gunduz, N.; Stejskal, E.O. Analysis of dimethacrylate copolymer (BisGMA and TEGDMA) network by DSC and 13C solution and solid-state NMR spectroscopy. Biomaterials 2000, 21, 1897–1903. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Stangel, I.; Ellis, T.H.; Zhu, X.X. A new method for quantifying the intensity of the C=C band of dimethacrylate dental monomers in their FTIR and Raman spectra. Biomaterials 2005, 26, 6440–6448. [Google Scholar] [CrossRef]
- Shin, W.S.; Li, X.F.; Schwartz, B.; Wunder, S.I.; Baran, G.R. Determination of the degree of cure of dental resins using Raman and FT-Raman spectroscopy. Dent. Mater. 1993, 9, 317–324. [Google Scholar] [CrossRef]
- Imazoto, S.; McCube, J.F.; Tarumi, H.; Ebara, A.; Ebisu, S. Degree of conversion of composites measured by DTA and FTIR. Dent. Mater. 2001, 17, 178–183. [Google Scholar] [CrossRef]
- Mc Cabe, J.F. Cure performance of light-activated composites by differential thermal analysis (DTA). Dent. Mater. 1985, 1, 231–234. [Google Scholar] [CrossRef]
- Borges, A.F.S.; Chase, M.A.; Guggiar, A.L.; Gonzales, M.J.; Ribeiro, A.R.; Pascon, F.M.; Zanatta, A.R. A critical review on the conversion degree of resin monomers by direct analyses. Braz. Dent. Sci. 2013, 16, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Jancar, J.; Wang, W.; Dibenedetto, A.T. On the heterogeneous structure of thermally cured bis-GMA/TEGDMA resins. J. Mater. Sci. Mater. Med. 2000, 9, 317–324. [Google Scholar]
- Loshack, S.; Fox, T.G. Cross-linked polymers. I. Factors influencing the efficiency of cross-linking in copolymers of methyl methacrylate and glycol dimethacrylates. J. Am. Chem. Soc. 1953, 75, 3544–3550. [Google Scholar] [CrossRef]
- Kwaśny, M.; Bombalska, A. Photopolymeryzation Kinetics of Methacrylate Dental of Resin by Transmission FTIR Method and Reliable Methodology. Biomed. J. Sci. Tech. Res. 2020, 30, 23072–23073. [Google Scholar]
- Brandt, W.C.; Schneider, L.F.; Frollini, E.; Correr-Sobrinho, L.; Sinhoreti, M.A. Effect of different photoinitiators and light-curing units on the degree of conversion of composites. Braz. Oral Res. 2010, 24, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, P. Curing efficiency of three light-emitting diode units at different curing profiles. Indian J. Dent. Res. 2016, 27, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Rueggeberg, F.A. Determination of resin cures using infrared analysis without an internal standard. Dent. Mater. 1994, 10, 282–286. [Google Scholar] [CrossRef]













| Materials | Components |
|---|---|
| Enamel Plus HRi Universal Enamel (UE2), GDF GmbH 61191—Rosbach, Germany | Monomers (20%) Diurethandimethacrylate Iso-propyliden-bis (2(3)-hydroxy-3(2)-4 (phenoxy)propyl-bis(methacrylate) (Bis-GMA) 4-Butandialdimethacrylate Filler (80%)—Glass filler (68%), average particle size 1.0 mm, Zirconia (12%), particle size 20 nm Activator—camphorquinone (CQ) |
| Enamel Plus HRi Universal Dentine (UD2), GDF GmbH 61191—Rosbach, Germany | Monomers (25%)—the same composition. Filler (75%)—Glass filler, medium particle size 0.7µm, silicon dioxide, medium size—0.04 µm Activator—camphorquinone (CQ) |
| DCi [%] (x) | SD (s) [%] | [%] | |
|---|---|---|---|
| 54.1 56.2 52.1 55.7 53.3 | 54.3 | 1.69 | 0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwaśny, M.; Bombalska, A.; Obroniecka, K. A Reliable Method of Measuring the Conversion Degrees of Methacrylate Dental Resins. Sensors 2022, 22, 2170. https://0-doi-org.brum.beds.ac.uk/10.3390/s22062170
Kwaśny M, Bombalska A, Obroniecka K. A Reliable Method of Measuring the Conversion Degrees of Methacrylate Dental Resins. Sensors. 2022; 22(6):2170. https://0-doi-org.brum.beds.ac.uk/10.3390/s22062170
Chicago/Turabian StyleKwaśny, Mirosław, Aneta Bombalska, and Karolina Obroniecka. 2022. "A Reliable Method of Measuring the Conversion Degrees of Methacrylate Dental Resins" Sensors 22, no. 6: 2170. https://0-doi-org.brum.beds.ac.uk/10.3390/s22062170