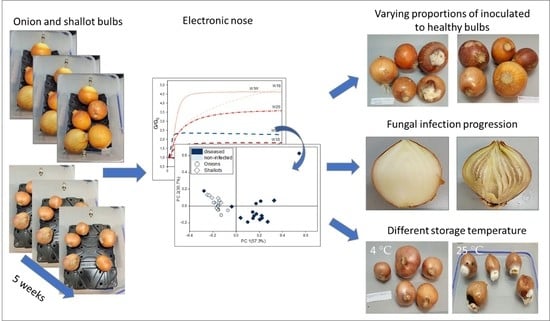
Preliminary Studies on Detection of Fusarium Basal Rot Infection in Onions and Shallots Using Electronic Nose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electronic Nose Measurements
2.3. Data Analysis
3. Results
3.1. Visual Examination of the Fusarium Basal Rot Symptoms
3.2. Sensor Array Responses
3.3. PCA
3.4. Classification of the Samples
- Classify samples with onion bulbs in terms of the proportion of infected to healthy bulbs (3 classes), i.e., non-infected (0%), mildly diseased (20–40% infected bulbs), and severe diseased (60–100% infected bulbs);
- Differentiate among samples containing non-infected (0%) and infected (100%) onion and shallot bulbs (two classes);
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buja, I.; Sabella, E.; Monteduro, A.G.; Chiriacò, M.S.; De Bellis, L.; Luvisi, A.; Maruccio, G. Advances in Plant Disease Detection and Monitoring: From Traditional Assays to in-Field Diagnostics. Sensors 2021, 21, 2129. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Ficke, A.; Aubertot, J.N.; Hollier, C. Crop Losses Due to Diseases and Their Implications for Global Food Production Losses and Food Security. Food Secur. 2012, 4, 519–537. [Google Scholar] [CrossRef]
- Ray, M.; Ray, A.; Dash, S.; Mishra, A.; Achary, K.G.; Nayak, S.; Singh, S. Fungal Disease Detection in Plants: Traditional Assays, Novel Diagnostic Techniques and Biosensors. Biosens. Bioelectron. 2017, 87, 708–723. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 Fungal Pathogens in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Michielse, C.B.; Rep, M. Pathogen Profile Update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Wang, A.; Islam, M.N.; Johansen, A.; Haapalainen, M.; Latvala, S.; Edelenbos, M. Pathogenic Fusarium oxysporum f. sp. Cepae Growing inside Onion Bulbs Emits Volatile Organic Compounds That Correlate with the Extent of Infection. Postharvest Biol. Technol. 2019, 152, 19–28. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A Review of Advanced Techniques for Detecting Plant Diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, L.R.; et al. Advanced Methods of Plant Disease Detection. A Review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Meents, A.K.; Mithöfer, A. Plant–Plant Communication: Is There a Role for Volatile Damage-Associated Molecular Patterns? Front. Plant Sci. 2020, 11, 583275. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. CRC. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Evans, P.; Ewen, R.J.; Gunson, H.E.; Jones, P.R.H.; Ratcliffe, N.M.; Spencer-Phillips, P.T.N. Gas Chromatography-Mass Spectrometry Analyses of Volatile Organic Compounds from Potato Tubers Inoculated with Phytophthora Infestans or Fusarium Coeruleum. Plant Pathol. 2001, 50, 489–496. [Google Scholar] [CrossRef]
- Cellini, A.; Biondi, E.; Blasioli, S.; Rocchi, L.; Farneti, B.; Braschi, I.; Savioli, S.; Rodriguez-Estrada, M.T.; Biasioli, F.; Spinelli, F. Early Detection of Bacterial Diseases in Apple Plants by Analysis of Volatile Organic Compounds Profiles and Use of Electronic Nose. Ann. Appl. Biol. 2016, 168, 409–420. [Google Scholar] [CrossRef]
- Vikram, A.; Prithiviraj, B.; Hamzehzarghani, H.; Kushalappa, A.C. Volatile Metabolite Profiling to Discriminate Diseases of McIntosh Apple Inoculated with Fungal Pathogens. J. Sci. Food Agric. 2004, 84, 1333–1340. [Google Scholar] [CrossRef]
- Cellini, A.; Biondi, E.; Buriani, G.; Farneti, B.; Rodriguez-Estrada, M.T.; Braschi, I.; Savioli, S.; Blasioli, S.; Rocchi, L.; Biasioli, F.; et al. Characterization of Volatile Organic Compounds Emitted by Kiwifruit Plants Infected with Pseudomonas Syringae Pv. Actinidiae and Their Effects on Host Defences. Trees-Struct. Funct. 2016, 30, 795–806. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Paramithiotis, S.; Drosinos, E.H.; Cammarisano, L.; Rogers, H.J.; Müller, C.T. Detection of Listeria Monocytogenes in Cut Melon Fruit Using Analysis of Volatile Organic Compounds. Food Microbiol. 2016, 54, 52–59. [Google Scholar] [CrossRef]
- Ficke, A.; Asalf, B.; Norli, H.R. Volatile Organic Compound Profiles From Wheat Diseases Are Pathogen-Specific and Can Be Exploited for Disease Classification. Front. Microbiol. 2022, 12, 803352. [Google Scholar] [CrossRef]
- Zainol Hilmi, N.H.; Idris, A.S.; Mohd Azmil, M.N. Headspace Solid-Phase Microextraction Gas Chromatography–Mass Spectrometry for the Detection of Volatile Organic Compounds Released from Ganoderma Boninense and Oil Palm Wood. For. Pathol. 2019, 49, e12531. [Google Scholar] [CrossRef]
- Cellini, A.; Blasioli, S.; Biondi, E.; Bertaccini, A.; Braschi, I.; Spinelli, F. Potential Applications and Limitations of Electronic Nose Devices for Plant Disease Diagnosis. Sensors 2017, 17, 2596. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Ling, P.; Zhu, H.; Keener, H.M. Plant Pest Detection Using an Artificial Nose System: A Review. Sensors 2018, 18, 378. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.W.; Bartlett, P.N. A Brief History of Electronic Noses. Sens. Actuators B Chem. 1994, 18, 211–220. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Horrillo, M.C. Advances in Artificial Olfaction: Sensors and Applications. Talanta 2014, 124, 95–105. [Google Scholar] [CrossRef] [PubMed]
- James, D.; Scott, S.M.; Ali, Z.; O’Hare, W.T. Chemical Sensors for Electronic Nose Systems. Microchim. Acta 2005, 149, 1–17. [Google Scholar] [CrossRef]
- Gutierrez-Osuna, R. Pattern Analysis for Machine Olfaction: A Review. IEEE Sens. J. 2002, 2, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.M.; James, D.; Ali, Z. Data Analysis for Electronic Nose Systems. Microchim. Acta 2006, 156, 183–207. [Google Scholar] [CrossRef]
- Covington, J.A.; Marco, S.; Persaud, K.C.; Schiffman, S.S.; Nagle, H.T. Artificial Olfaction in the 21st Century. IEEE Sens. J. 2021, 21, 12969–12990. [Google Scholar] [CrossRef]
- Burlachenko, J.; Kruglenko, I.; Snopok, B.; Persaud, K. Sample Handling for Electronic Nose Technology: State of the Art and Future Trends. TrAC-Trends Anal. Chem. 2016, 82, 222–236. [Google Scholar] [CrossRef] [Green Version]
- Röck, F.; Barsan, N.; Weimar, U. Electronic Nose: Current Status and Future Trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st Century Technique for Food Control: Electronic Noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef]
- Mohd Ali, M.; Hashim, N.; Abd Aziz, S.; Lasekan, O. Principles and Recent Advances in Electronic Nose for Quality Inspection of Agricultural and Food Products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic Nose as a Tool for Monitoring the Authenticity of Food. A Review. Food Anal. Methods 2017, 10, 1800–1816. [Google Scholar] [CrossRef] [Green Version]
- John, A.T.; Murugappan, K.; Nisbet, D.R.; Tricoli, A. An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring. Sensors 2021, 21, 2271. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic Noses: Powerful Tools in Meat Quality Assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Varnamkhasti, M.; Apetrei, C.; Lozano, J.; Anyogu, A. Potential Use of Electronic Noses, Electronic Tongues and Biosensors as Multisensor Systems for Spoilage Examination in Foods. Trends Food Sci. Technol. 2018, 80, 71–92. [Google Scholar] [CrossRef]
- Sanaeifar, A.; ZakiDizaji, H.; Jafari, A.; Guardia, M. de la Early Detection of Contamination and Defect in Foodstuffs by Electronic Nose: A Review. TrAC-Trends Anal. Chem. 2017, 97, 257–271. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic Noses for Environmental Monitoring Applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef]
- Wilson, A.D. Applications of Electronic-Nose Technologies for Noninvasive Early Detection of Plant, Animal and Human Diseases. Chemosensors 2018, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Wasilewski, T.; Gębicki, J. Emerging Strategies for Enhancing Detection of Explosives by Artificial Olfaction. Microchem. J. 2021, 164, 106025. [Google Scholar] [CrossRef]
- Wilson, A.D. Diverse Applications of Electronic-Nose Technologies in Agriculture and Forestry. Sensors 2013, 13, 2295–2348. [Google Scholar] [CrossRef] [Green Version]
- Rusinek, R.; Jelen, H.; Malaga-Tobola, U.; Molenda, M.; Gancarz, M. Influence of Changes in the Level of Volatile Compounds Emitted during Rapeseed Quality Degradation on the Reaction of MOS Type Sensor-Array. Sensors 2020, 20, 3135. [Google Scholar] [CrossRef]
- Borowik, P.; Adamowicz, L.; Tarakowski, R.; Wacławik, P.; Oszako, T.; Ślusarski, S.; Tkaczyk, M. Development of a Low-Cost Electronic Nose for Detection of Pathogenic Fungi and Applying It to Fusarium oxysporum and Rhizoctonia Solani. Sensors 2021, 21, 5868. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, W.; Zhu, N.; Mao, S.; Tu, K. Early Detection and Classification of Pathogenic Fungal Disease in Post-Harvest Strawberry Fruit by Electronic Nose and Gas Chromatography-Mass Spectrometry. Food Res. Int. 2014, 62, 162–168. [Google Scholar] [CrossRef]
- Makarichian, A.; Chayjan, R.A.; Ahmadi, E.; Zafari, D. Early Detection and Classification of Fungal Infection in Garlic (A. Sativum) Using Electronic Nose. Comput. Electron. Agric. 2022, 192, 106575. [Google Scholar] [CrossRef]
- Eifler, J.; Martinelli, E.; Santonico, M.; Capuano, R.; Schild, D.; Di Natale, C. Differential Detection of Potentially Hazardous Fusarium Species in Wheat Grains by an Electronic Nose. PLoS ONE 2011, 6, e21026. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Tian, H.; Sun, J.; Wan, C. Electronic Nose-Based Technique for Rapid Detection and Recognition of Moldy Apples. Sensors 2019, 19, 1526. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhao, N.; Zhou, D.; Sun, Y.; Sun, K.; Pan, L.; Tu, K. Discrimination and Growth Tracking of Fungi Contamination in Peaches Using Electronic Nose. Food Chem. 2018, 262, 226–234. [Google Scholar] [CrossRef]
- Li, C.; Schmidt, N.E.; Gitaitis, R. Detection of Onion Postharvest Diseases by Analyses of Headspace Volatiles Using a Gas Sensor Array and GC-MS. LWT-Food Sci. Technol. 2011, 44, 1019–1025. [Google Scholar] [CrossRef]
- Konduru, T.; Rains, G.C.; Li, C. Detecting Sour Skin Infected Onions Using a Customized Gas Sensor Array. J. Food Eng. 2015, 160, 19–27. [Google Scholar] [CrossRef]
- Li, C.; Gitaitis, R.; Tollner, B.; Sumner, P.; MacLean, D. Onion Sour Skin Detection Using a Gas Sensor Array and Support Vector Machine. Sens. Instrum. Food Qual. Saf. 2009, 3, 193–202. [Google Scholar] [CrossRef]
- Taylor, A.; Vágány, V.; Jackson, A.C.; Harrison, R.J.; Rainoni, A.; Clarkson, J.P. Identification of Pathogenicity-Related Genes in Fusarium oxysporum f. sp. Cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef] [Green Version]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Amylsis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Sokolova, M.; Lapalme, G. A Systematic Analysis of Performance Measures for Classification Tasks. Inf. Process. Manag. 2009, 45, 427–437. [Google Scholar] [CrossRef]
- Berrueta, L.A.; Alonso-salces, R.M. Supervised Pattern Recognition in Food Analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef]
- Oliveri, P.; Casolino, M.C.; Forina, M. Chemometric Brains for Artificial Tongues. In Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 61, pp. 57–117. [Google Scholar]
- Cortes, C.; Vapnik, V. Supprot-Vector Networks. Mach. Learn. 1995, 297, 273–297. [Google Scholar] [CrossRef]
- Le, D.; Audenaert, K.; Haesaert, G. Fusarium Basal Rot: Profile of an Increasingly Important Disease in Allium spp. Trop. Plant Pathol. 2021, 46, 241–253. [Google Scholar] [CrossRef]
- Cramer, C.S. Breeding and Genetics of Fusarium Basal Rot Resistance in Onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Wang, A.; Haapalainen, M.; Latvala, S.; Edelenbos, M.; Johansen, A. Discriminant Analysis of Volatile Organic Compounds of Fusarium oxysporum f. sp. Cepae and Fusarium Proliferatum Isolates from Onions as Indicators of Fungal Growth. Fungal Biol. 2018, 122, 1013–1022. [Google Scholar] [CrossRef]
- Vikram, A.; Hamzehzarghani, H.; Kushalappa, A.C. Volatile Metabolites from the Headspace of Onion Bulbs Inoculated with Postharvest Pathogens as a Tool for Disease Discrimination. Can. J. Plant Pathol. 2005, 27, 194–203. [Google Scholar] [CrossRef]








| No | Producent’s Designation | General Description |
|---|---|---|
| 1 | W1C | aromatic compounds |
| 2 | W5S | broad range of compounds, nitrogen oxides |
| 3 | W3C | ammonia, aromatic compounds |
| 4 | W6S | Hydrogen |
| 5 | W5C | alkenes, aromatic compounds |
| 6 | W1S | methane, broad range of compounds |
| 7 | W1W | sulphur-organic compounds |
| 8 | W2S | alcohols, broad range of organic compounds |
| 9 | W2W | aromatic compounds, sulphur organic compounds |
| 10 | W3S | alkenes, aliphatic organic compounds |
| 3 Class Classification | 2 Class Classification | |||||
|---|---|---|---|---|---|---|
| Accuracy | Recall | F1 Score | Accuracy | Recall | F1 Score | |
| LDA | 89.60% | 0.90 | 0.92 | 93.80% | 0.88 | 0.93 |
| LDA 5 sensors | 66.70% | 0.67 | 0.74 | 90.60% | 0.81 | 0.90 |
| SVM linear | 79.20% | 0.79 | 0.84 | 87.50% | 0.75 | 0.86 |
| SVM optimized | 87.50% | 0.83 | 0.87 | 96.90% | 0.94 | 0.97 |
| k-NN optimized | 89.60% | 0.90 | 0.92 | 93.80% | 0.88 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labanska, M.; van Amsterdam, S.; Jenkins, S.; Clarkson, J.P.; Covington, J.A. Preliminary Studies on Detection of Fusarium Basal Rot Infection in Onions and Shallots Using Electronic Nose. Sensors 2022, 22, 5453. https://0-doi-org.brum.beds.ac.uk/10.3390/s22145453
Labanska M, van Amsterdam S, Jenkins S, Clarkson JP, Covington JA. Preliminary Studies on Detection of Fusarium Basal Rot Infection in Onions and Shallots Using Electronic Nose. Sensors. 2022; 22(14):5453. https://0-doi-org.brum.beds.ac.uk/10.3390/s22145453
Chicago/Turabian StyleLabanska, Malgorzata, Sarah van Amsterdam, Sascha Jenkins, John P. Clarkson, and James A. Covington. 2022. "Preliminary Studies on Detection of Fusarium Basal Rot Infection in Onions and Shallots Using Electronic Nose" Sensors 22, no. 14: 5453. https://0-doi-org.brum.beds.ac.uk/10.3390/s22145453