Application of Gas Chromatography Mass Spectrometry in Tar Analysis from Underground Gasification
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Gas Products and Temperature History
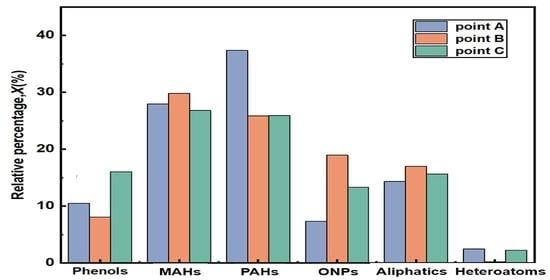
3.2. Tar Behaviors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- An, N.; Zagorscak, R.; Thomas, H.R.; Gao, W. A numerical investigation into the environmental impact of underground coal gasification technology based on a coupled thermal-hydro-chemical model. J. Clean. Prod. 2021, 290, 125181. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Ren, B.; Ding, K. The Layout of the Combustion Cavity and the Fracture Evolution of the Overlying Rock during the Process of Underground Coal Gasification. Geofluids 2022, 2022, 9264959. [Google Scholar] [CrossRef]
- Mandapati, R.N.; Ghodke, P.K. Kinetic modeling of Indian lignites pyrolysis in the context of underground coal gasification (UCG). Fuel 2021, 283, 118939. [Google Scholar] [CrossRef]
- Xie, J.; Xin, L.; Hu, X.M.; Cheng, W.M.; Liu, W.T.; Wang, Z.G. Technical application of safety and cleaner production technology by underground coal gasification in China. J. Clean. Prod. 2020, 250, 119487. [Google Scholar] [CrossRef]
- Mallett, C.W. Environmental controls for underground coal gasification. J. Power Energy 2018, 232, 47–55. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification—Part I: Field demonstrations and process performance. Prog. Energy Combust. Sci. 2018, 67, 158–187. [Google Scholar] [CrossRef]
- Li, J.; Yao, X.; Xu, K.; Ge, J.; Yang, D.; Fan, B. Numerical investigation of a process model integrating gasification and tar removal. Biomass Convers. Biorefinery 2021, 1–15. [Google Scholar] [CrossRef]
- Xu, M.; Xin, L.; Liu, W.; Hu, X.; Cheng, W.; Li, C.; Wang, Z. Study on the physical properties of coal pyrolysis in underground coal gasification channel. Powder Technol. 2020, 376, 573–592. [Google Scholar] [CrossRef]
- Niu, M.; Wang, R.; Ma, W.; Guo, W.; Liu, H.; Liu, S. Methane formation mechanism during pressurized pyrolysis of coal core in the context of deep underground coal gasification. Fuel 2022, 324, 124668. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, D.; Li, Y.; Tian, H.; Liang, J. Influence of scale and atmosphere on the pyrolysis properties of large-scale bituminous coal. J. Anal. Appl. Pyrolysis 2021, 158, 105060. [Google Scholar] [CrossRef]
- Grabowski, J.; Korczak, K.; Tokarz, A. Aquatic risk assessment based on the results of research on minewaters as a part of a pilot underground coal gasification process. Process Saf. Environ. Prot. 2021, 148, 548–558. [Google Scholar] [CrossRef]
- Ding, R.; Sun, Q.; Xue, S.; Shi, Q.; Ge, Z.; Li, D. Experimental study on acoustic emission characteristics of high-temperature thermal damage in an oxygen-rich environment of long flame coal. J. Therm. Anal. Calorim. 2022, 147, 11391–11400. [Google Scholar] [CrossRef]
- Aloisi, I.; Zoccali, M.; Tranchida, P.; Mondello, L. Analysis of Organic Sulphur Compounds in Coal Tar by Using Comprehensive Two-Dimensional Gas Chromatography-High Resolution Time-of-Flight Mass Spectrometry. Separations 2020, 7, 26. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Liu, J.; Wang, Z.; Gong, X.; Guo, L.; Song, W. Pyrolysis of Liulin coal simulated by GPU-Based ReaxFF MD with cheminformatics analysis. Energy Fuels 2014, 28, 522–534. [Google Scholar] [CrossRef]
- Zhang, K.; Sang, S.; Ma, M.; Zhou, X.; Liu, C.; Shen, G. Permeability response characteristics of primary undeformed coal and tectonically deformed coal under loading−unloading conditions in Huainan coalfield, China. ACS Omega 2022, 7, 37485–37498. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Y. Role of coal deformation energy in coal and gas outburst: A review. Fuel 2023, 332, 126019. [Google Scholar] [CrossRef]
- Ding, Z.; Feng, X.; Wang, E.; Wei, Q.; Zhao, X.; Hu, Q. Acoustic emission response and evolution of precracked coal in the meta-instability stage under graded loading. Eng. Geol. 2023, 312, 106930. [Google Scholar] [CrossRef]
- Haefelfinger, P. Limits of the internal standard technique in chromatography. J. Chromatogr. A 1981, 218, 73–81. [Google Scholar] [CrossRef]
- Prando, D.; Ail, S.; Chiaramonti, D.; Baratieri, M.; Dasappa, S. Characterisation of the producer gas from an open top gasifier: Assessment of different tar analysis approaches. Fuel 2016, 181, 566–572. [Google Scholar] [CrossRef]
- Dong, M.; Feng, L.; Zhou, Q.; Zhou, S.; Xu, X.; Qin, B. Spatial and temporal evolution of tar during ex-situ underground coal gasification. Fuel 2022, 317, 123423. [Google Scholar] [CrossRef]
- Xin, H.; Zhou, B.; Tian, W.; Qi, X.; Zheng, M.; Lu, W.; Yang, H.; Zhong, X.; Wang, D. Pyrolytic stage evolution mechanism of Zhundong coal based on reaction consistency analysis of mono/multi molecular models. Fuel 2023, 333, 126371. [Google Scholar] [CrossRef]
- Murakami, T.; Yasuda, H.; Norisada, K. Comparison of tar components in syngas generated by gasification conditions of Lignite in a fluidized bed gasifier. Energy Fuels 2018, 32, 1110–1144. [Google Scholar] [CrossRef]
- Winchell, L.; Ross, J.; Brose, D.; Pluth, T.; Fonoll, X.; Norton, J.W., Jr.; Bell, K.Y. Pyrolysis and gasification at water resource recovery facilities: Status of the industry. Water Environ. Res. 2022, 94, e10701. [Google Scholar] [CrossRef] [PubMed]









| Tar Sample | Top 5 Chemicals | Molecular Formula | Percentage (%) | Structural Formula |
|---|---|---|---|---|
| A | (1) | C15H13N | 15.19 |  |
| (2) | C10H8 | 5.87 |  | |
| (3) | C12H15NO4 | 3.14 |  | |
| (4) | C11H10 | 2.38 |  | |
| (5) | C10H6ClNO2 | 2.07 |  | |
| B | (1) | C15H13N | 12.8 |  |
| (2) | C15H13N | 3.35 |  | |
| (3) | C3H7NO | 3.25 |  | |
| (4) | C9H13N | 2.93 |  | |
| (5) | C10H8 | 2.4 |  | |
| C | (1) | C15H13N | 8.48 |  |
| (2) | C15H13N | 5.59 |  | |
| (3) | C12H16O | 4.48 |  | |
| (4) | C12H17NO2 | 3.54 |  | |
| (5) | C9H11N3O3 | 2.36 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Liu, J.; Xin, H.; Pang, J. Application of Gas Chromatography Mass Spectrometry in Tar Analysis from Underground Gasification. Separations 2023, 10, 12. https://0-doi-org.brum.beds.ac.uk/10.3390/separations10010012
Feng L, Liu J, Xin H, Pang J. Application of Gas Chromatography Mass Spectrometry in Tar Analysis from Underground Gasification. Separations. 2023; 10(1):12. https://0-doi-org.brum.beds.ac.uk/10.3390/separations10010012
Chicago/Turabian StyleFeng, Lele, Jie Liu, Haihui Xin, and Jiabao Pang. 2023. "Application of Gas Chromatography Mass Spectrometry in Tar Analysis from Underground Gasification" Separations 10, no. 1: 12. https://0-doi-org.brum.beds.ac.uk/10.3390/separations10010012