Metal–Organic Frameworks as Key Materials for Solid-Phase Microextraction Devices—A Review
Abstract
:1. Overview on Metal–Organic Framework
2. Metal–Organic Frameworks in Analytical Separations
3. MOFs in On-Fiber Solid-Phase Microextraction (f-SPME)
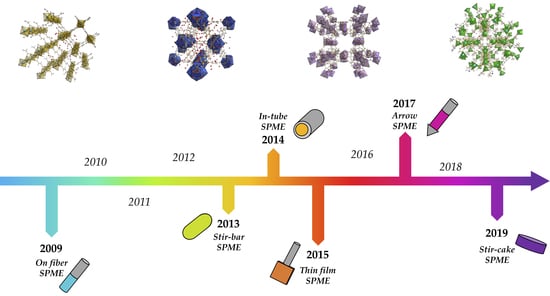
3.1. Overwiev on Commercial f-SPME Devices
3.2. Preparation of MOF-Based f-SPME Devices
3.3. Analytical Performance of MOF-Based f-SPME Devices
4. MOFs in On-Arrow-Fiber Solid-Phase Microextraction (af-SPME)
5. MOFs in In-Tube Solid-Phase Microextraction (it-SPME)
6. MOFs in Thin-Film Solid-Phase Microextraction (tf-SPME)
7. MOFs in Stir-Bar (sb-SPME) and Stir-Cake Solid-Phase Microextraction (sc-SPME)
8. Comparison with Other MOF-Based Extraction Methods
9. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| af-SPME | arrow fiber solid-phase microextraction |
| ALD | atomic layer deposition |
| BMA-EDMA | butyl methacrylate-ethylene dimethacrylate |
| CE | capillary electrophoresis |
| CEC | capillary electrochromatography |
| d-µ-SPE | dispersive solid-phase microextraction |
| DAD | diode array detection |
| DI | direct immersion |
| ECD | electron capture detection |
| f-SPME | on-fiber solid-phase microextraction |
| FID | flame ionization detection |
| GC | gas chromatography |
| FPD | flame photometric detection |
| HS | headspace |
| IL | ionic liquid |
| IRMOF | isoreticular metal–organic framework |
| it-SPME | in-tube solid-phase microextraction |
| LC | liquid chromatography |
| LOD | limit of detection |
| m-d-µ-SPE | magnetic-assisted miniaturized solid-phase extraction |
| MIP | molecularly imprinted polymer |
| MNP | magnetic nanoparticle |
| MOF | metal–organic framework |
| MS | mass spectrometry |
| MS/MS | tandem mass spectrometry |
| OCP | organochlorine pesticide |
| OPP | organophosphorus pesticide |
| PAH | polycyclic aromatic hydrocarbon |
| PAN | polyacrylonitrile |
| PCB | polychlorinated biphenyl |
| PDMS | polydimethylsiloxane |
| PEEK | polyetheretherketone |
| PS | polystyrene |
| PVC | polyvinylchloride |
| PVDF | polyvinylidene difluoride |
| sb-SPME | stir-bar solid-phase microextraction |
| SBU | secondary building unit |
| sc-SPME | stir-cake solid-phase microextraction |
| SESI | secondary electrospray ionization |
| SPME | solid-phase microextraction |
| tf-SPME | thin-film solid-phase microextraction |
| µ-SPE | miniaturized solid-phase extraction |
References
- Rowsell, J.L.C.; Yaghi, O.M. Metal-Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A Review on Metal-Organic Frameworks: Synthesis and Applications. Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary Building Units as the Turning Point in the Development of the Reticular Chemistry of MOFs. Sci. Adv. 2018, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M. Reticular Chemistry—Construction, Properties, and Precision Reactions of Frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- The Cambridge Crystallographic Data Center (CCDC). Available online: https://www.ccdc.cam.ac.uk/ (accessed on 25 July 2019).
- Millange, F.; Serre, C. Synthesis, Structure Determination and Properties of MIL-53as and MIL-53ht: The First CrIII Hybrid Inorganic—Organic Microporous Solids: CrIII(OH)·{O2C–C6H4–CO2}·{HO2C–C6H4–CO2H}x. Chem. Commun. 2002, 8, 822–823. [Google Scholar] [CrossRef]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2 (H2O)3](N). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef]
- Akporiayer, D.E.; Fjellvag, H.; Halvorsen, E.N.; Hustveit, J.; Karlsson, A.; Lillerud, K.P. The synthesis and structure solution of UiO-7, a new molecular sieve. Chem. Commun. 1996, 5, 601–602. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pino, V.; Ayala, J.H.; Ruiz-Pérez, C.; Vallcorba, O.; Afonso, A.M.; Pasán, J. A Green Metal-Organic Framework to Monitor Water Contaminants. RSC Adv. 2018, 8, 31304–31310. [Google Scholar] [CrossRef]
- Küsgens, P.; Rose, M.; Senkovska, I.; Fröde, H.; Henschel, A.; Siegle, S.; Kaskel, S. Characterization of Metal-Organic Frameworks by Water Adsorption. Microporous Mesoporous Mater. 2009, 120, 325–330. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, C.; Ji, Y.; You, X.; Peng, S.; Lee, G. [Co5(im)10·2MB]∞: A Metal-Organic Open.Framework with Zeolite-Like Topology. Angew. Chem. 2002, 114, 1442–1444. [Google Scholar] [CrossRef]
- Feng, D.; Gu, Z.Y.; Li, J.R.; Jiang, H.L.; Wei, Z.; Zhou, H.C. Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal-Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts. Angew. Chem. Int. Ed. 2012, 51, 10307–10310. [Google Scholar] [CrossRef] [PubMed]
- Julien, P.A.; Mottillo, C.; Friščić, T. Metal-Organic Frameworks Meet Scalable and Sustainable Synthesis. Green Chem. 2017, 19, 2729–2747. [Google Scholar] [CrossRef]
- Ding, L.; Yazaydin, A.O. Hydrogen and Methane Storage in Ultrahigh Surface Area Metal-Organic Frameworks. Microporous Mesoporous Mater. 2013, 182, 185–190. [Google Scholar] [CrossRef]
- Hönicke, I.M.; Senkovska, I.; Bon, V.; Baburin, I.A.; Bönisch, N.; Raschke, S.; Evans, J.D.; Kaskel, S. Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials. Angew. Chem. Int. Ed. 2018, 57, 13780–13783. [Google Scholar] [CrossRef]
- Howarth, A.J.; Peters, A.W.; Vermeulen, N.A.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Best Practices for the Synthesis, Activation, and Characterization of Metal−organic Frameworks. Chem. Mater. 2017, 29, 26–39. [Google Scholar] [CrossRef]
- Xue, D.X.; Wang, Q.; Bai, J. Amide-Functionalized Metal–Organic Frameworks: Syntheses, Structures and Improved Gas Storage and Separation Properties. Coord. Chem. Rev. 2019, 378, 2–16. [Google Scholar] [CrossRef]
- Xu, C.; Fang, R.; Luque, R.; Chen, L.; Li, Y. Functional Metal–Organic Frameworks for Catalytic Applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- Anik, Ü.; Timur, S.; Dursun, Z. Metal Organic Frameworks in Electrochemical and Optical Sensing Platforms: A Review. Microchim. Acta 2019, 186, 18–24. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Lau, J.; Fan, W.; Li, Q.; Zhang, C.; Huang, P.; Chen, X. Recent Progress in Nanoscale Metal-Organic Frameworks for Drug Release and Cancer Therapy. Nanomedicine 2019, 14, 1343–1365. [Google Scholar] [CrossRef] [PubMed]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-Organic Frameworks for Energy Storage Devices: Batteries and Supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Termopoli, V. Metal–Organic Frameworks in Solid-Phase Extraction Procedures for Environmental and Food Analyses. Chromatographia 2019, 82, 1191–1205. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; González-Hernández, P.; Pino, V.; Pasán, J.; Afonso, A.M. Metal-Organic Frameworks as Novel Sorbents in Dispersive-Based Microextraction Approaches. Trends Anal. Chem. 2017, 90, 114–134. [Google Scholar] [CrossRef]
- González-Hernández, P.; Gutiérrez-Serpa, A.; Rocío-Bautista, P.; Pasán, J.; Ayala, J.H.; Pino, V. Micro-solid Phase Extraction using MOFs. In Metal Organic Frameworks; Mittal, V., Ed.; Central West Publishing: Orange, Australia, 2019; pp. 99–136. [Google Scholar]
- Pacheco-Fernández, I.; González-Hernández, P.; Pasán, J.; Ayala, J.H.; Pino, V. The Rise of Metal–Organic Frameworks in Analytical Chemistry. In Handbook of Smart Materials in Analytical Chemistry; De la Guardia, M., Esteve-Turrillas, F.A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2019; pp. 463–502. [Google Scholar]
- Maya, F.; Palomino Cabello, C.; Figuerola, A.; Turnes Palomino, G.; Cerdà, V. (Eds.) Immobilization of Metal–Organic Frameworks on Supports for Sample Preparation and Chromatographic Separation. Chromatographia 2019, 82, 361–375. [Google Scholar]
- Liang, Y.; Zhang, L.; Zhang, Y. Well-Defined Materials for Chromatographic Separation. Annu. Rev. Anal. Chem. 2019, 12, 451–473. [Google Scholar] [CrossRef]
- Faraji, M.; Yamini, Y.; Gholami, M. Recent Advances and Trends in Applications of Solid-Phase Extraction Techniques in Food and Environmental Analysis. Chromatographia 2019, 82, 1207–1249. [Google Scholar] [CrossRef]
- Maya, F.; Palomino Cabello, C.; Frizzarin, R.M.; Estela, J.M.; Turnes Palomino, G.; Cerdà, V. Magnetic Solid-Phase Extraction Using Metal-Organic Frameworks (MOFs) and Their Derived Carbons. Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are Metal-Organic Frameworks Able to Provide a New Generation of Solid-Phase Microextraction Coatings?—A Review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Li, H.; Bai, Y.; Liu, H. Metal-Organic Frameworks as Advanced Sorbents in Sample Preparation for Small Organic Analytes. Coord. Chem. Rev. 2019, 397, 1–13. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-Organic Frameworks in Green Analytical Chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Yan, X.P.; Kim, K.N.; Wang, S.W.; Liu, M.G. Exploration of Coordination Polymer as Sorbent for Flow Injection Solid-Phase Extraction on-Line Coupled with High-Performance Liquid Chromatography for Determination of Polycyclic Aromatic Hydrocarbons in Environmental Materials. J. Chromatogr. A 2006, 1116, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Yang, C.X.; Yan, X.P. Zeolite Imidazolate Framework-8 as Sorbent for on-Line Solid-Phase Extraction Coupled with High-Performance Liquid Chromatography for the Determination of Tetracyclines in Water and Milk Samples. J. Chromatogr. A 2013, 1304, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, C.; Yan, Z.; Cai, Q.; Yao, S. Evaluation of metal-organic framework 5 as a new SPE material for the determination of polycyclic aromatic hydrocarbons in environmental waters. J. Sep. Sci. 2013, 36, 1283–1290. [Google Scholar] [CrossRef]
- Ge, D.; Lee, H.K. Sonication-Assisted Emulsification Microextraction Combined with Vortex-Assisted Porous Membrane-Protected Micro-Solid-Phase Extraction Using Mixed Zeolitic Imidazolate Frameworks 8 as Sorbent. J. Chromatogr. A 2012, 1263, 1–6. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K.H. Metal-Organic Frameworks as Advanced Sorbents for the Extraction and Determination of Pollutants from Environmental, Biological, and Food Media. Trends Anal. Chem. 2017, 97, 65–82. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of Geometries and Coating Materials in Solid Phase Microextraction: Opportunities, Limitations, and Future Perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An Overview of the Most Common Lab-Made Coating Materials in Solid Phase Microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef]
- Helin, A.; Rönkkö, T.; Parshintsev, J.; Hartonen, K.; Schilling, B.; Läubli, T.; Riekkola, M.L. Solid Phase Microextraction Arrow for the Sampling of Volatile Amines in Wastewater and Atmosphere. J. Chromatogr. A 2015, 1426, 56–63. [Google Scholar] [CrossRef]
- Costa Queiroz, M.E.; Donizeti de Souza, I.; Marchioni, C. Current Advances and Applications of In-Tube Solid-Phase Microextraction. Trends Anal. Chem. 2019, 111, 261–278. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyacı, E. Thin Film Microextraction: Towards Faster and More Sensitive Microextraction. Trends Anal. Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- David, F.; Ochiai, N.; Sandra, P. Two Decades of Stir Bar Sorptive Extraction: A Retrospective and Future Outlook. Trends Anal. Chem. 2019, 112, 102–111. [Google Scholar] [CrossRef]
- Niu, J.; Li, Z.; Yang, H.; Ye, C.; Chen, C.; Li, D.; Xu, J.; Fan, L. A Water Resistant Solid-Phase Microextraction Fiber with High Selectivity Prepared by a Metal Organic Framework with Perfluorinated Pores. J. Chromatogr. A 2016, 1441, 16–23. [Google Scholar] [CrossRef]
- Xiao, Z.; He, M.; Chen, B.; Hu, B. Polydimethylsiloxane/Metal-Organic Frameworks Coated Stir Bar Sorptive Extraction Coupled to Gas Chromatography-Flame Photometric Detection for the Determination of Organophosphorus Pesticides in Environmental Water Samples. Talanta 2016, 156–157, 126–133. [Google Scholar] [CrossRef]
- Shih, Y.H.; Wang, K.Y.; Singco, B.; Lin, C.H.; Huang, H.Y. Metal-Organic Framework-Polymer Composite as a Highly Efficient Sorbent for Sulfonamide Adsorption and Desorption: Effect of Coordinatively Unsaturated Metal Site and Topology. Langmuir 2016, 32, 11465–11473. [Google Scholar] [CrossRef]
- Gao, G.; Li, S.; Li, S.; Zhao, L.; Wang, T.; Hou, X. Development and application of vortex-assisted membrane extraction based on metal-organic framework mixed-matrix membrane for the analysis of estrogens in human urine. Anal. Chim. Acta 2018, 1023, 35–43. [Google Scholar] [CrossRef]
- Lan, H.; Rönkkö, T.; Parshintsev, J.; Hartonen, K.; Gan, N.; Sakeye, M.; Sarfraz, J.; Riekkola, M.L. Modified Zeolitic Imidazolate Framework-8 as Solid-Phase Microextraction Arrow Coating for Sampling of Amines in Wastewater and Food Samples Followed by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2017, 1486, 76–85. [Google Scholar] [CrossRef]
- Du, F.; Sun, L.; Tan, W.; Wei, Z.; Nie, H.; Huang, Z.; Ruan, G.; Li, J. Magnetic Stir Cake Sorptive Extraction of Trace Tetracycline Antibiotics in Food Samples: Preparation of Metal–Organic Framework-Embedded PolyHIPE Monolithic Composites, Validation and Application. Anal. Bioanal. Chem. 2019, 411, 2239–2248. [Google Scholar] [CrossRef]
- Merck Group SPME Fiber Assemblies. Available online: https://www.sigmaaldrich.com/analytical-chromatography/analytical-products.html?TablePage=9645337 (accessed on 25 July 2019).
- Restek PAL SPME Fibers. Product Details. Available online: https://www.restek.com/catalog/view/47352 (accessed on 25 July 2019).
- Shirey, R.E. SPME Commercial Devices and Fibre Coatings. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 99–133. [Google Scholar]
- Souza-Silva, É.A.; Gionfriddo, E.; Pawliszyn, J. A Critical Review of the State of the Art of Solid-Phase Microextraction of Complex Matrices I. Environmental Analysis. Trends Anal. Chem. 2015, 71, 224–235. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Gionfriddo, E.; Pawliszyn, J. A Critical Review of the State of the Art of Solid-Phase Microextraction of Complex Matrices II. Food Analysis. Trends Anal. Chem. 2015, 71, 236–248. [Google Scholar] [CrossRef]
- Souza-Silva, É.A.; Reyes-Garcés, N.; Gómez-Ríos, G.A.; Boyaci, E.; Bojko, B.; Pawliszyn, J. A Critical Review of the State of the Art of Solid-Phase Microextraction of Complex Matrices III. Bioanalytical and Clinical Applications. Trends Anal. Chem. 2015, 71, 249–264. [Google Scholar] [CrossRef]
- USEPA (U.S. Environmental Protection Agency). Method 8272, 2007; EPA: Washington, DC, USA, 2007.
- American Society for Testing and Materials. D 6438, 2005; EPA: Washington, DC, USA, 2005. [Google Scholar]
- Cui, X.Y.; Gu, Z.Y.; Jiang, D.Q.; Li, Y.; Wang, H.F.; Yan, X.P. In Situ Hydrothermal Growth of Metal-Organic Framework 199 Films on Stainless Steel Fibers for Solid-Phase Microextraction of Gaseous Benzene Homologues. Anal. Chem. 2009, 81, 9771–9777. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, C.; Lin, Y.; Tong, P.; Zhang, L. In Situ Solvothermal Synthesis of Metal-Organic Framework Coated Fiber for Highly Sensitive Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. J. Chromatogr. A 2016, 1436, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Huang, C.; Feng, Z.; Chen, H.; Tong, P.; Wu, X.; Zhang, L. Metal-Organic Framework-Coated Stainless Steel Fiber for Solid-Phase Microextraction of Polychlorinated Biphenyls. J. Chromatogr. A 2018, 1570, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Pan, D.; Sun, Y.; Guo, Y.; Wu, Z. Thin Metal Organic Frameworks Coatings by Cathodic Electrodeposition for Solid-Phase Microextraction and Analysis of Trace Exogenous Estrogens in Milk. Anal. Chim. Acta 2016, 937, 53–60. [Google Scholar] [CrossRef]
- Zhang, S.; Du, Z.; Li, G. Metal-Organic Framework-199/Graphite Oxide Hybrid Composites Coated Solid-Phase Microextraction Fibers Coupled with Gas Chromatography for Determination of Organochlorine Pesticides from Complicated Samples. Talanta 2013, 115, 32–39. [Google Scholar] [CrossRef]
- Chen, X.F.; Zang, H.; Wang, X.; Cheng, J.G.; Zhao, R.S.; Cheng, C.G.; Lu, X.Q. Metal-Organic Framework MIL-53(Al) as a Solid-Phase Microextraction Adsorbent for the Determination of 16 Polycyclic Aromatic Hydrocarbons in Water Samples by Gas Chromatography-Tandem Mass Spectrometry. Analyst 2012, 137, 5411–5419. [Google Scholar] [CrossRef]
- Shang, H.B.; Yang, C.X.; Yan, X.P. Metal-Organic Framework UiO-66 Coated Stainless Steel Fiber for Solid-Phase Microextraction of Phenols in Water Samples. J. Chromatogr. A 2014, 1357, 165–171. [Google Scholar] [CrossRef]
- Mondal, S.; Xu, J.; Chen, G.; Huang, S.; Huang, C.; Yin, L.; Ouyang, G. Solid-Phase Microextraction of Antibiotics from Fish Muscle by Using MIL-101(Cr)NH 2 -Polyacrylonitrile Fiber and Their Identification by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2019, 1047, 62–70. [Google Scholar] [CrossRef]
- Zhang, G.; Zang, X.; Li, Z.; Wang, C.; Wang, Z. Polydimethylsiloxane/Metal-Organic Frameworks Coated Fiber for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in River and Lake Water Samples. Talanta 2014, 129, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. A Nanocomposite Consisting of Graphene Oxide, Zeolite Imidazolate Framework 8, and a Molecularly Imprinted Polymer for (Multiple) Fiber Solid Phase Microextraction of Sterol and Steroid Hormones Prior to Their Quantitation by HPLC. Microchim. Acta 2019, 186, 129. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Salmi, L.D.; Rönkkö, T.; Parshintsev, J.; Jussila, M.; Hartonen, K.; Kemell, M.; Riekkola, M.L. Integrated Atomic Layer Deposition and Chemical Vapor Reaction for the Preparation of Metal Organic Framework Coatings for Solid-Phase Microextraction Arrow. Anal. Chim. Acta 2018, 1024, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Lirio, S.; Chen, Y.T.; Lin, C.H.; Huang, H.Y. A Novel Hybrid Metal-Organic Framework-Polymeric Monolith for Solid-Phase Microextraction. Chem. A Eur. J. 2014, 20, 3317–3321. [Google Scholar] [CrossRef]
- Hu, C.; He, M.; Chen, B.; Zhong, C.; Hu, B. Polydimethylsiloxane/Metal-Organic Frameworks Coated Stir Bar Sorptive Extraction Coupled to High Performance Liquid Chromatography-Ultraviolet Detector for the Determination of Estrogens in Environmental Water Samples. J. Chromatogr. A 2013, 1310, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, M.; Chen, B.; Zhong, C.; Hu, B. Sorptive Extraction Using Polydimethylsiloxane/Metal-Organic Framework Coated Stir Bars Coupled with High Performance Liquid Chromatography-Fluorescence Detection for the Determination of Polycyclic Aromatic Hydrocarbons in Environmental Water Samples. J. Chromatogr. A 2014, 1356, 45–53. [Google Scholar] [CrossRef]
- Lin, S.; Gan, N.; Qiao, L.; Zhang, J.; Cao, Y.; Chen, Y. Magnetic Metal-Organic Frameworks Coated Stir Bar Sorptive Extraction Coupled with GC-MS for Determination of Polychlorinated Biphenyls in Fish Samples. Talanta 2015, 144, 1139–1145. [Google Scholar] [CrossRef]
- Yang, J.H.; Cui, C.X.; Qu, L.B.; Chen, J.; Zhou, X.M.; Zhang, Y.P. Preparation of a Monolithic Magnetic Stir Bar for the Determination of Sulfonylurea Herbicides Coupled with HPLC. Microchem. J. 2018, 141, 369–376. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Ding, W.; Li, G. Multilayer Interparticle Linking Hybrid MOF-199 for Noninvasive Enrichment and Analysis of Plant Hormone Ethylene. Anal. Chem. 2014, 86, 3533–3540. [Google Scholar] [CrossRef]
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Preparation and Characterization of Magnetic Metal–Organic Framework Nanocomposite as Solid-Phase Microextraction Fibers Coupled with High-Performance Liquid Chromatography for Determination of Non-Steroidal Anti-Inflammatory Drugs in Biological Fluids and Tablet Formulation Samples. Microchem. J. 2019, 144, 270–284. [Google Scholar]
- Sun, S.; Huang, L.; Xiao, H.; Shuai, Q.; Hu, S. In Situ Self-Transformation Metal into Metal-Organic Framework Membrane for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Talanta 2019, 202, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, S.; Xu, J.; Yin, L.; Sun, F.; Zhou, N.; Ouyang, G. Fabrication of 8-Aminocaprylic Acid Doped UIO-66 as Sensitive Solid-Phase Microextraction Fiber for Nitrosamines. Talanta 2018, 178, 629–635. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, M.; Wang, X.; Luo, C.; Feng, J. A Nanospherical Metal–Organic Framework UiO-66 for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Chromatographia 2018, 81, 1053–1061. [Google Scholar] [CrossRef]
- Xie, L.; Liu, S.; Han, Z.; Jiang, R.; Liu, H.; Zhu, F.; Zeng, F.; Su, C.; Ouyang, G. Preparation and Characterization of Metal-Organic Framework MIL-101(Cr)-Coated Solid-Phase Microextraction Fiber. Anal. Chim. Acta 2015, 853, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Zhang, G.; Chang, Q.; Zhang, X.; Wang, C.; Wang, Z. Metal Organic Framework MIL-101 Coated Fiber for Headspace Solid Phase Microextraction of Volatile Aromatic Compounds. Anal. Methods 2015, 7, 918–923. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Yang, C.X.; Yan, X.P. Fabrication of Metal-Organic Framework MIL-88B Films on Stainless Steel Fibers for Solid-Phase Microextraction of Polychlorinated Biphenyls. J. Chromatogr. A 2014, 1334, 1–8. [Google Scholar] [CrossRef]
- Jia, Y.; Su, H.; Wang, Z.; Wong, Y.L.E.; Chen, X.; Wang, M.; Chan, T.W.D. Metal-Organic Framework@Microporous Organic Network as Adsorbent for Solid-Phase Microextraction. Anal. Chem. 2016, 88, 9364–9367. [Google Scholar] [CrossRef]
- Lv, F.; Gan, N.; Huang, J.; Hu, F.; Cao, Y.; Zhou, Y.; Dong, Y.; Zhang, L.; Jiang, S. A Poly-Dopamine Based Metal-Organic Framework Coating of the Type PDA-MIL-53(Fe) for Ultrasound-Assisted Solid-Phase Microextraction of Polychlorinated Biphenyls Prior to Their Determination by GC-MS. Microchim. Acta 2017, 184, 2561–2568. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Q.; Li, Z.; Wang, W.; Zang, X.; Wang, C.; Wang, Z. Solid Phase Microextraction of Phthalic Acid Esters from Vegetable Oils Using Iron (III)-Based Metal-Organic Framework/Graphene Oxide Coating. Food Chem. 2018, 263, 258–264. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, G.; Li, L.; Wang, X.; Li, N.; Zhao, R.S.; Lin, J. Facile Fabrication of MIL-96 as Coating Fiber for Solid-Phase Microextraction of Trihalomethanes and Halonitromethanes in Water Samples. Chem. Eng. J. 2018, 350, 240–247. [Google Scholar] [CrossRef]
- Abolghasemi, M.M.; Yousefi, V.; Piryaei, M. Synthesis of a Metal-Organic Framework Confined in Periodic Mesoporous Silica with Enhanced Hydrostability as a Novel Fiber Coating for Solid-Phase Microextraction. J. Sep. Sci. 2015, 38, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Q.; Wang, W.; Wang, C.; Wang, Z. Covalent Bonding of Metal-Organic Framework-5/Graphene Oxide Hybrid Composite to Stainless Steel Fiber for Solid-Phase Microextraction of Triazole Fungicides from Fruit and Vegetable Samples. J. Agric. Food Chem. 2016, 64, 2792–2801. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Gan, N.; Zhang, J.; Qiao, L.; Chen, Y.; Cao, Y. Aptamer-Functionalized Stir Bar Sorptive Extraction Coupled with Gas Chromatography-Mass Spectrometry for Selective Enrichment and Determination of Polychlorinated Biphenyls in Fish Samples. Talanta 2016, 149, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ai, Y.; Zeng, B.; Zhao, F. In Situ Solvothermal Growth of Metal-Organic Framework-Ionic Liquid Functionalized Graphene Nanocomposite for Highly Efficient Enrichment of Chloramphenicol and Thiamphenicol. J. Chromatogr. A 2016, 1427, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Gu, Z.Y.; Wang, H.F.; Yan, X.P. Metal-Organic-Framework-Based Tandem Molecular Sieves as a Dual Platform for Selective Microextraction and High-Resolution Gas Chromatographic Separation of n -Alkanes in Complex Matrixes. Anal. Chem. 2011, 83, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Q.; Yan, X.P. Covalent Bonding of Zeolitic Imidazolate Framework-90 to Functionalized Silica Fibers for Solid-Phase Microextraction. Chem. Commun. 2013, 49, 2142–2144. [Google Scholar] [CrossRef]
- Yu, L.Q.; Wang, L.Y.; Su, F.H.; Hao, P.Y.; Wang, H.; Lv, Y.K. A Gate-Opening Controlled Metal-Organic Framework for Selective Solid-Phase Microextraction of Aldehydes from Exhaled Breath of Lung Cancer Patients. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, F.; Huang, W.; He, H.; Hu, J.; Sun, C.; Xian, Q.; Yang, S. Sol–Gel Based Metal-Organic Framework Zeolite Imidazolate Framework-8 Fibers for Solid-Phase Microextraction of Nitro Polycyclic Aromatic Hydrocarbons and Polycyclic Aromatic Hydrocarbons in Water Samples. J. Chromatogr. A 2019. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal-Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal-Organic Frameworks. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef]
- He, C.T.; Tian, J.Y.; Liu, S.Y.; Ouyang, G.; Zhang, J.P.; Chen, X.M. A Porous Coordination Framework for Highly Sensitive and Selective Solid-Phase Microextraction of Non-Polar Volatile Organic Compounds. Chem. Sci. 2013, 4, 351–356. [Google Scholar] [CrossRef]
- Zheng, J.; Li, S.; Wang, Y.; Li, L.; Su, C.; Liu, H.; Zhu, F.; Jiang, R.; Ouyang, G. In Situ Growth of IRMOF-3 Combined with Ionic Liquids to Prepare Solid-Phase Microextraction Fibers. Anal. Chim. Acta 2014, 829, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.A.; Yang, F.; Liu, Z.C.; Liu, Q.K.; Dong, Y. Bin. A Porous Cd(Ii)-MOF-Coated Quartz Fiber for Solid-Phase Microextraction of BTEX. J. Mater. Chem. A 2014, 2, 13868–13872. [Google Scholar] [CrossRef]
- Li, Q.L.; Wang, X.; Chen, X.F.; Wang, M.L.; Zhao, R.S. In Situ Hydrothermal Growth of Ytterbium-Based Metal-Organic Framework on Stainless Steel Wire for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Environmental Samples. J. Chromatogr. A 2015, 1415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.H.; Yu, J.; Fu, Y.Y.; Zhou, P.X. In Situ Hydrothermal Growth of a Dual-Ligand Metal-Organic Framework Film on a Stainless Steel Fiber for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Environmental Water Samples. RSC Adv. 2016, 6, 14042–14048. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Su, H.; Elaine Wong, Y.L.; Chen, X.; Dominic Chan, T.W.; Chen, Q. In Situ Hydrothermal Growth of a Zirconium-Based Porphyrinic Metal-Organic Framework on Stainless Steel Fibers for Solid-Phase Microextraction of Nitrated Polycyclic Aromatic Hydrocarbons. Microchim. Acta 2017, 184, 3809–3815. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, C.; Tong, P.; Feng, Z.; Wu, X.; Zhang, L. Moisture Stable Ni-Zn MOF/g-C 3 N 4 Nanoflowers: A Highly Efficient Adsorbent for Solid-Phase Microextraction of PAHs. J. Chromatogr. A 2018, 1556, 37–46. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Guo, C.; Li, Y. Metal Azolate Framework-66-Coated Fiber for Headspace Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. J. Chromatogr. A 2019, 1584, 57–63. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.; Zheng, J.; Xu, J.; Jiang, R.; Shen, Y.; Jiang, J.; Zhu, F.; Su, C.; Ouyang, G. Isoreticular Bio-MOF 100–102 Coated Solid-Phase Microextraction Fibers for Fast and Sensitive Determination of Organic Pollutants by the Pore Structure Dominated Mechanism. Analyst 2015, 140, 4384–4387. [Google Scholar] [CrossRef]
- Wang, G.; Lei, Y.; Song, H. Exploration of Metal-Organic Framework MOF-177 Coated Fibers for Headspace Solid-Phase Microextraction of Polychlorinated Biphenyls and Polycyclic Aromatic Hydrocarbons. Talanta 2015, 144, 369–374. [Google Scholar] [CrossRef]
- Wei, S.; Lin, W.; Xu, J.; Wang, Y.; Liu, S.; Zhu, F.; Liu, Y.; Ouyang, G. Fabrication of a Polymeric Composite Incorporating Metal-Organic Framework Nanosheets for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Water Samples. Anal. Chim. Acta 2017, 971, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xie, L.; Hu, Q.; Yang, H.; Pan, G.; Zhu, F.; Yang, S.; Ouyang, G. A Tri-Metal Centered Metal-Organic Framework for Solid-Phase Microextraction of Environmental Contaminants with Enhanced Extraction Efficiency. Anal. Chim. Acta 2017, 987, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, H.; Yamini, Y.; Masoomi, M.Y.; Morsali, A. Nanostructured Metal-Organic Frameworks, TMU-4, TMU-5, and TMU-6, as Novel Adsorbents for Solid Phase Microextraction of Polycyclic Aromatic Hydrocarbons. New J. Chem. 2017, 41, 12035–12043. [Google Scholar] [CrossRef]
- Bagheri, H.; Amanzadeh, H.; Yamini, Y.; Masoomi, M.Y.; Morsali, A.; Salar-Amoli, J.; Hassan, J. A Nanocomposite Prepared from a Zinc-Based Metal-Organic Framework and Polyethersulfone as a Novel Coating for the Headspace Solid-Phase Microextraction of Organophosphorous Pesticides. Microchim. Acta 2018, 185, 62. [Google Scholar] [CrossRef]
- Wang, J.; Du, Q.; You, X.; Lv, Y.; Bi, W.; Li, H.; Chen, D.D.Y. Solvent-Free High-Throughput Analysis of Herbicides in Environmental Water. Anal. Chim. Acta 2019, 1071, 8–16. [Google Scholar] [CrossRef]
- Zang, X.; Zhang, X.; Chang, Q.; Li, S.; Wang, C.; Wang, Z. Metal–Organic Framework UiO-67-Coated Fiber for the Solid-Phase Microextraction of Nitrobenzene Compounds from Water. J. Sep. Sci. 2016, 39, 2770–2776. [Google Scholar] [CrossRef]
- Niu, J.; Zhao, X.; Jin, Y.; Yang, G.; Li, Z.; Wang, J.; Zhao, R.; Li, Z. Determination of Aromatic Amines in the Urine of Smokers Using a Porous Organic Framework (JUC-Z2)-Coated Solid-Phase Microextraction Fiber. J. Chromatogr. A 2018, 1555, 37–44. [Google Scholar] [CrossRef]
- Bagheri, H.; Javanmardi, H.; Abbasi, A.; Banihashemi, S. A Metal Organic Framework-Polyaniline Nanocomposite as a Fiber Coating for Solid Phase Microextraction. J. Chromatogr. A 2016, 1431, 27–35. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X.; Yuan, D. Multiple Monolithic Fiber Solid-Phase Microextraction: A New Extraction Approach for Aqueous Samples. J. Chromatogr. A 2014, 1345, 29–36. [Google Scholar] [CrossRef]
- PAL SYSTEM: PAL Smart SPME Arrows. Available online: https://www.palsystem.com/index.php?id=822 (accessed on 25 July 2019).
- Yuan, Y.; Lin, X.; Li, T.; Pang, T.; Dong, Y.; Zhuo, R.; Wang, Q.; Cao, Y.; Gan, N. A Solid Phase Microextraction Arrow with Zirconium Metal–Organic Framework/Molybdenum Disulfide Coating Coupled with Gas Chromatography–Mass Spectrometer for the Determination of Polycyclic Aromatic Hydrocarbons in Fish Samples. J. Chromatogr. A 2019, 1592, 9–18. [Google Scholar] [CrossRef]
- Eisert, R.; Pawliszyn, J. Automated In-Tube Solid-Phase Microextraction Coupled to High-Performance Liquid Chromatography. Anal. Chem. 1997, 69, 3140–3147. [Google Scholar] [CrossRef]
- Moliner-Martinez, Y.; Herráez-Hernández, R.; Verdú-Andrés, J.; Molins-Legua, C.; Campíns-Falcó, P. Recent Advances of In-Tube Solid-Phase Microextraction. Trends Anal. Chem. 2015, 71, 205–213. [Google Scholar] [CrossRef]
- Fernández-Amado, M.; Prieto-Blanco, M.C.; López-Mahía, P.; Muniategui-Lorenzo, S.; Prada-Rodríguez, D. Strengths and Weaknesses of In-Tube Solid-Phase Microextraction: A Scoping Review. Anal. Chim. Acta 2016, 906, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Lirio, S.; Liu, W.L.; Lin, C.L.; Lin, C.H.; Huang, H.Y. Aluminum Based Metal-Organic Framework-Polymer Monolith in Solid-Phase Microextraction of Penicillins in River Water and Milk Samples. J. Chromatogr. A 2016, 1428, 236–245. [Google Scholar] [CrossRef]
- Ling, X.; Chen, Z. Immobilization of Zeolitic Imidazolate Frameworks with Assist of Electrodeposited Zinc Oxide Layer and Application in Online Solid-Phase Microextraction of Sudan Dyes. Talanta 2019, 192, 142–146. [Google Scholar] [CrossRef]
- Wilcockson, J.B.; Gobas, F.A.P.C. Thin-Film Solid-Phase Extraction to Measure Fugacities of Organic Chemicals with Low Volatility in Biological Samples. Environ. Sci. Technol. 2001, 35, 1425–1431. [Google Scholar] [CrossRef]
- Jiang, R.; Pawliszyn, J. Thin-Film Microextraction Offers Another Geometry for Solid-Phase Microextraction. Trends Anal. Chem. 2012, 39, 245–253. [Google Scholar] [CrossRef]
- Carasek, E.; Merib, J. Membrane-Based Microextraction Techniques in Analytical Chemistry: A Review. Anal. Chim. Acta 2015, 880, 8–25. [Google Scholar] [CrossRef]
- Tascon, M.; Gómez-Ríos, G.A.; Reyes-Garcés, N.; Poole, J.; Boyacl, E.; Pawliszyn, J. High-Throughput Screening and Quantitation of Target Compounds in Biofluids by Coated Blade Spray-Mass Spectrometry. Anal. Chem. 2017, 89, 8421–8428. [Google Scholar] [CrossRef]
- Mohammadi, V.; Jafari, M.T.; Saraji, M. Flexible/Self-Supported Zeolitic Imidazolate Framework-67 Film as an Adsorbent for Thin-Film Microextraction. Microchem. J. 2019, 146, 98–105. [Google Scholar] [CrossRef]
- Ghani, M.; Font Picó, M.F.; Salehinia, S.; Palomino Cabello, C.; Maya, F.; Berlier, G.; Saraji, M.; Cerdà, V.; Turnes Palomino, G. Metal-Organic Framework Mixed-Matrix Disks: Versatile Supports for Automated Solid-Phase Extraction Prior to Chromatographic Separation. J. Chromatogr. A 2017, 1488, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xu, H. Development of a Novel Polystyrene/Metal-Organic Framework-199 Electrospun Nanofiber Adsorbent for Thin Film Microextraction of Aldehydes in Human Urine. Talanta 2017, 162, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.S.; Cohen, S.M. In Situ Modification of Metal-Organic Frameworks in Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Ren, Y.; Xu, H. Metal–Organic Framework 199 Film as a Novel Adsorbent of Thin-Film Extraction. Chromatographia 2015, 78, 621–629. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir Bar Sorptive Extraction (SBSE), a Novel Extraction Technique for Aqueous Samples: Theory and Principles. J. Microcol. Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Lin, F.; Yuan, D. Novel Extraction Approach for Liquid Samples: Stir Cake Sorptive Extraction Using Monolith. J. Sep. Sci. 2011, 34, 2145–2151. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized Solid-Phase Extraction Techniques. Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Prieto, A.; Basauri, O.; Rodil, R.; Usobiaga, A.; Fernández, L.A.; Etxebarria, N.; Zuloaga, O. Stir-Bar Sorptive Extraction: A View on Method Optimisation, Novel Applications, Limitations and Potential Solutions. J. Chromatogr. A 2010, 1217, 2642–2666. [Google Scholar] [CrossRef]
- Automated Sample Preparation, GC/MS & LC/MS Solutions by GERSTEL. Available online: http://www.gerstel.com/ (accessed on 25 July 2019).
- Hu, Y.; Lian, H.; Zhou, L.; Li, G. In Situ Solvothermal Growth of Metal-Organic Framework-5 Supported on Porous Copper Foam for Noninvasive Sampling of Plant Volatile Sulfides. Anal. Chem. 2015, 87, 406–412. [Google Scholar] [CrossRef]
- You, L.; He, M.; Chen, B.; Hu, B. One-pot synthesis of zeolitic imidazolate framework-8/poly(methyl methacrylate-ethyleneglycol dimethacrylate) monolith coating for stir bat sorptive extraction of phytohormones from fruit samples followed by high performance liquid chromatography-ultraviolet detection. J. Chromatogr. A 2017, 1524, 57–65. [Google Scholar]
- Wang, Y.; Jia, M.; Wu, X.; Wang, T.; Wang, J.; Hou, X. PEG Modified Column MIL-101(Cr)/PVA Cryogel as a Sorbent in Stir Bar Solid Phase Extraction for Determination of Non-Steroidal Anti-Inflammatory Drugs in Water Samples. Microchem. J. 2019, 146, 214–219. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, W.; Liao, X.; Wang, X.; Chen, Z. Covalent Immobilization of Metal Organic Frameworks onto Chemical Resistant Poly(Ether Ether Ketone) Jacket for Stir Bar Extraction. Anal. Chim. Acta 2018, 1025, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Ghoreishi, S.M.; Azamati, M. In-Situ Growth of Zeolitic Imidazole Framework-67 on Nanoporous Anodized Aluminum Bar as Stir-Bar Sorptive Extraction Sorbent for Determining Caffeine. J. Chromatogr. A 2018, 1577, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, Z.; Wang, S.; Hong, S.; Li, H.; Zhang, C.; Shao, Y.; She, Y.; Jin, F.; Jin, M.; et al. Metal-organic framework UiO-66 for rapid dispersive solid phase extraction of neonicotinoid insecticides in water samples. J. Chromatogr. B 2018, 1077, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Lin, Y.; Wang, Z.; Wong, Y.-L.E.; Chen, X.; Chan, T.-W.D. Magnetic metal–organic framework–titanium dioxide nanocomposite as adsorbent in the magnetic solid-phase extraction of fungicides from environmental water samples. J. Chromatogr. A 2016, 1466, 21–28. [Google Scholar] [CrossRef]
- Dai, X.; Jia, X.; Zhao, P.; Wang, T.; Wang, J.; Huang, P.; He, L.; Hou, X. A combined experimental/computational study on metal-organic framework MIL-101(Cr) as a SPE for the determination of sulphonamides in environmental water samples coupling with UPLC-MS/MS. Talanta 2016, 154, 581–588. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Gao, G.; Zhao, P.; Lu, N.; Lun, X.; Hou, X. Magnetic solid phase extraction of non-steroidal anti-inflammatory drugs from water samples using a metal organic framework of type Fe3O4/MIL-101(Cr), and their quantitation by UPLC-MS/MS. Microchim. Acta 2017, 184, 2981–2990. [Google Scholar] [CrossRef]







| MOF | Support | Additive* | Size (Length/Diameter/Thickness) | Preparation Method | Sample/Analyte* (Number) | Analytical Method* | Extraction Time (min) | RSDmax/RSDbatch a | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| On-fiber solid-phase microextraction (f-SPME) | |||||||||
| HKUST-1(Cu) | stainless steel wire | – | –/–/40 µm | in-situ growth | indoor air/benzene derivatives (7) | HS mode and GC-FID | 20 | 7.7/9.4 | [61] |
| UiO-66(Zr) | fused silica fiber | – | –/–/25 µm | in-situ growth | water and soil/PAHs (10) | DI mode and GC-MS | 20 | 8.2/8.9 | [62] |
| ZIF-90(Zn) | stainless steel wire | – | –/–/30.5 µm | in-situ growth | water, soil, and vegetables/PCBs (6) | DI mode and GC-MS | 40 | 5.5/9.1 | [63] |
| E-MOF-5(Zn) | stainless steel wire | – | –/–/12.5 µm | electro-deposition | milk/hormones (4) | DI mode and LC-DAD | 30 | 9.4/6.1 | [64] |
| HKUST-1(Cu) | fused silica fiber | graphite oxide | –/–/40 µm | immersion in the composite | water and soil/OCPs (8) | HS mode and GC-ECD | 40 | 8.8/12.8 | [65] |
| MIL-53(Al) | stainless steel wire | epoxy glue as adhesive | –/–/50 µm | attachment with adhesive | water/PAHs (16) | HS mode and GC-MS/MS | 50 | 12.5/13.9 | [66] |
| UiO-66(Zr) | stainless steel wire | epoxy glue as adhesive | –/–/150 µm | attachment with adhesive | water/phenols (6) | HS mode and GC-FID | 50 | 6.2/10.1 | [67] |
| MIL-101-NH2(Cr) | quartz | PAN as adhesive | –/–/120 µm | attachment with adhesive | fish/antibiotics (6) | In-vivo and LC-MS/MS | 10 | 6.8/9.5 | [68] |
| MIL-101(Cr) | stainless steel wire | PDMS | –/–/70 µm | sol–gel | water/PAHs (5) | HS mode and GC-MS | 20 | 9.3/13.8 | [69] |
| ZIF-8(Zn) | fiber bundle with 4 monoliths | graphene oxide and MIP | 3 cm/0.35 cm/– | mold polymerization | food/hormones (5) | DI mode and LC-MS | 30 | 4.1/5.2 | [70] |
| On-arrow-fiber solid-phase microextraction (af-SPME) | |||||||||
| ZIF-8(Zn) | arrow steel rod | PVC as adhesive | 2 cm/–/70 µm | attachment with adhesive | wastewater, fish and mushroom/amines (2) | HS mode and GC-MS | 5 | 10.3/15.6 | [51] |
| Fe-BDC(Fe) | arrow steel rod | - | 2 cm/–/2 µm | atomic layer deposition and conversion | wastewater/chloro-phenols (8) | HS mode and GC-MS | 30 | 23.1/– | [71] |
| In-tube solid-phase microextraction (it-SPME) | |||||||||
| MIL-101(Cr) | capillary tube | BMA-EDMA and IL [C6mim][BF4] | 10 cm/0.8 mm/– | microwave assisted polymerization | water/drugs (6) | CEC-UV-Vis | 34 | 5.2/– | [72] |
| MIL-53(Al) | capillary tube | BMA-EDMA and IL [C6mim][BF4] | 10 cm/0.8 mm/– | microwave assisted polymerization | water/sulfonamides (7) | CE-UV-Vis | 36 | 6.4/5.3 | [49] |
| MOF | Support | Additive* | Size (Length/Diameter/Thickness) | Preparation Method | Sample/Analyte* (Number) | Analytical Method* | Extraction Time (min) | RSDmax/RSDbatch a | Ref. |
| Thin film solid-phase microextraction (tf-SPME) | |||||||||
| MIL-53(Al) | – | PVDF | 2 cm/–/– | spreading | urine/estrogens (4) | LC-FD | 45 | 11.4/– | [50] |
| Stir-bar solid-phase microextraction (sb-SPME) | |||||||||
| IRMOF-3(Zn) | capillary glass bar | PDMS | 2 cm/–/100 µm | sol–gel | water/estrogens (7) | LC-UV | 55 | 10.2/16.1 | [73] |
| MIL-53-NH2(Al) | capillary glass bar | PDMS | 2 cm/–/125 µm | sol–gel | water/PAHs (15) | LC-FD | 30 | 11.7/16.9 | [74] |
| MOF-5(Fe) | Nd-Fe-B rod | MNP Fe3O4@NH2 | 1 cm/–/– | magnetic interaction | fish/PCBs (6) | GC-MS | 33 | 4.3/– | [75] |
| MIL-101-NH2(Cr) | capillary glass bar | PDMS | 2 cm/–/100 µm | sol–gel | water/OPPs (6) | GC-FPD | 35 | 10.7/9.2 | [48] |
| UiO-66-NH2(Zr) | – | Nd-Fe-B powder, 4-VP | 2 cm/30 mm/– | thermal polymerization | soil and water/herbicides (5) | LC-UV | 60 | 13.8/9.5 | [76] |
| Extraction Method* | MOF | Amount of Sorbent a | Volume of Sample | Desorption | Additional Steps | Extraction Time | Reuse of the Sorbent/Device | Analytical Technique* | LOD (ng·L−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Analytical application 1: determination of pesticides in waters | ||||||||||
| µ-dSPE | UiO-66(Zr) | 40 mg | 5 mL | liquid (1 mL acetone) | centrifugation, evaporation and reconstitution | ~20 min | 10 times | LC-MS/MS | 20–400 | [144] |
| m-µ-dSPE | ZIF-8(Zn) | 0.5 mg | 10 mL | liquid (1 mL methanol) | evaporation and reconstitution | ~45 min | No | LC-MS/MS | 0.19–1.20 | [145] |
| f-SPME (HS mode) | HKUST-1(Cu) | – × 40 µm | 25 mL | thermal (280 °C) | – | ~45 min | 140 times | GC-ECD | 2.8–6.9 | [65] |
| tf-SPME | ZIF-67(Zn) | 1 cm × 80 µm | 15 mL | thermal (220 °C) | – | ~22 min | No | SESI/MS | 100 | [129] |
| sb-SPME | MIL-101-NH2(Cr) | 2 cm × 100 µm | 10 mL | liquid (50 µL acetone) | – | ~35 min | 50 times | GC-FPD | 43–85 | [48] |
| Analytical application 2: determination of drugs in waters | ||||||||||
| µ-SPE | MIL-101(Cr) | 40 mg | 60 mL | liquid (4 mL methanol) | evaporation and reconstitution | ~60 min | No | LC-MS/MS | 30–80 | [146] |
| m-µ-dSPE | MIL-101(Cr) | 30 mg | 50 mL | liquid (200 µL acetonitrile) | evaporation and reconstitution | ~30 min | No | LC-MS/MS | 3–60 | [147] |
| f-SPME (HS mode) | HKUST-1(Cu) | 1 cm × 30 µm | 5 mL | liquid (2 mL acetonitrile:H2O 1:1) | evaporation and reconstitution | ~60 min | 110 times | LC-UV-Vis | 30–50 | [78] |
| it-SPME | MIL-101(Cr) | 3 cm × – | 2 mL | liquid (200 µL methanol) | – | ~35 min | 45 times | CEC-UV-Vis | 1200–4500 | [72] |
| sb-SPME | MIL-101(Cr) | 1 cm × – | 10 mL | liquid (1 mL acetonitrile) | evaporation and reconstitution | ~80 min | 4 times | LC-MS/MS | 11–35 | [141] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–Organic Frameworks as Key Materials for Solid-Phase Microextraction Devices—A Review. Separations 2019, 6, 47. https://0-doi-org.brum.beds.ac.uk/10.3390/separations6040047
Gutiérrez-Serpa A, Pacheco-Fernández I, Pasán J, Pino V. Metal–Organic Frameworks as Key Materials for Solid-Phase Microextraction Devices—A Review. Separations. 2019; 6(4):47. https://0-doi-org.brum.beds.ac.uk/10.3390/separations6040047
Chicago/Turabian StyleGutiérrez-Serpa, Adrián, Idaira Pacheco-Fernández, Jorge Pasán, and Verónica Pino. 2019. "Metal–Organic Frameworks as Key Materials for Solid-Phase Microextraction Devices—A Review" Separations 6, no. 4: 47. https://0-doi-org.brum.beds.ac.uk/10.3390/separations6040047