Analytical Determination of Allergenic Fragrances in Indoor Air
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gas Chromatography–Mass Spectrometry
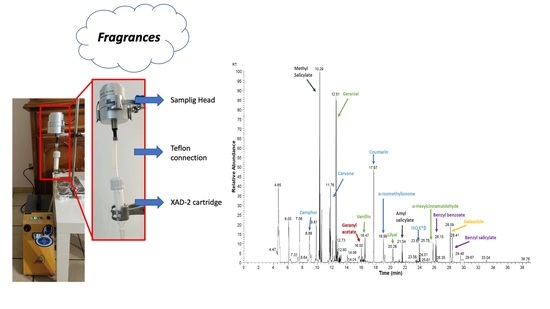
2.3. Sampling System and Analytical Procedure
2.4. Indoor Sampling
3. Results and Discussion
3.1. Method Setting and Effectiveness
3.1.1. GC Analysis
3.1.2. Sampling and Extraction Optimization
3.1.3. Blank Evaluation
3.1.4. Effectiveness of the Method
3.1.5. Breakthrough Evaluation
3.2. Concentrations of Fragrances in the Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Settimo, G.; Manigrasso, M.; Avino, P. Indoor air quality: A focus on the european legislation and state-of-the-art research in Italy. Atmosphere 2020, 11, 370. [Google Scholar] [CrossRef] [Green Version]
- Maroni, M.; Axelrad, R.; Bacaloni, A. NATO’s efforts to set indoor air quality guidelines and standards. Am. Ind. Hyg. Assoc. J. 1995, 56, 499–508. [Google Scholar] [CrossRef]
- Takaoka, M.; Norbäck, D. The Indoor Environment in Schools, Kindergartens and Day Care Centres. In Indoor Environmental Quality and Health Risk toward Healthier Environment for All; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 9789813291812. [Google Scholar]
- Baloch, R.M.; Maesano, C.N.; Christoffersen, J.; Banerjee, S.; Gabriel, M.; Csobod, É.; de Oliveira Fernandes, E.; Annesi-Maesano, I.; Szuppinger, P.; Prokai, R.; et al. Indoor air pollution, physical and comfort parameters related to schoolchildren’s health: Data from the European SINPHONIE study. Sci. Total Environ. 2020, 739, 139870. [Google Scholar] [CrossRef] [PubMed]
- Nehr, S.; Hösen, E.; Tanabe, S. Ichi Emerging developments in the standardized chemical characterization of indoor air quality. Environ. Int. 2017, 98, 233–237. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A state–of–the-art review on indoor air pollution and strategies for indoor air pollution control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef] [PubMed]
- Wolkoff, P.; Nielsen, G.D. Effects by inhalation of abundant fragrances in indoor air—An overview. Environ. Int. 2017, 101, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Basketter, D.; Kimber, I. Fragrance sensitisers: Is inhalation an allergy risk? Regul. Toxicol. Pharmacol. 2015, 73, 897–902. [Google Scholar] [CrossRef]
- Nawaz, T.; Sengupta, S. Chapter 4—Contaminants of Emerging Concern: Occurrence, Fate, and Remediation. In Advances in Water Purification Techniques; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128147917. [Google Scholar]
- Yadav, D.; Rangabhashiyam, S.; Verma, P.; Singh, P.; Devi, P.; Kumar, P.; Hussain, C.M.; Gaurav, G.K.; Kumar, K.S. Environmental and health impacts of contaminants of emerging concerns: Recent treatment challenges and approaches. Chemosphere 2021, 272, 129492. [Google Scholar] [CrossRef]
- Enyoh, C.E.; Verla, A.W.; Qingyue, W.; Ohiagu, F.O.; Chowdhury, A.H.; Enyoh, E.C.; Chowdhury, T.; Verla, E.N.; Chinwendu, U.P. An overview of emerging pollutants in air: Method of analysis and potential public health concern from human environmental exposure. Trends Environ. Anal. Chem. 2020, 28, e00107. [Google Scholar] [CrossRef]
- Christensson, J.B.; Hagvall, L.; Karlberg, A.T. Fragrance allergens, overview with a focus on recent developments and understanding of abiotic and biotic activation. Cosmetics 2016, 3, 19. [Google Scholar] [CrossRef]
- Bickers, D.R.; Calow, P.; Greim, H.A.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.H.; Sipes, I.G.; Smith, R.L.; Tagami, H. The safety assessment of fragrance materials. Regul. Toxicol. Pharmacol. 2003, 37, 218–273. [Google Scholar] [CrossRef]
- Patel, S.; Homaei, A.; Sharifian, S. Need of the hour: To raise awareness on vicious fragrances and synthetic musks. Environ. Dev. Sustain. 2020, 23 (Suppl. 3), 4764–4781. [Google Scholar] [CrossRef]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieck, S.; Olsson, O.; Kümmerer, K.; Klaschka, U. Fragrance allergens in household detergents. Regul. Toxicol. Pharmacol. 2018, 97, 163–169. [Google Scholar] [CrossRef]
- Chen, J.; Møller, K.H.; Wennberg, P.O.; Kjaergaard, H.G. Unimolecular Reactions following Indoor and Outdoor Limonene Ozonolysis. J. Phys. Chem. A 2021, 125, 669–680. [Google Scholar] [CrossRef]
- Nørgaard, A.W.; Kofoed-Sørensen, V.; Mandin, C.; Ventura, G.; Mabilia, R.; Perreca, E.; Cattaneo, A.; Spinazzè, A.; Mihucz, V.G.; Szigeti, T.; et al. Ozone-initiated terpene reaction products in five European offices: Replacement of a floor cleaning agent. Environ. Sci. Technol. 2014, 48, 13331–13339. [Google Scholar] [CrossRef]
- Lamas, J.P.; Sanchez-Prado, L.; Garcia-Jares, C.; Llompart, M. Determination of fragrance allergens in indoor air by active sampling followed by ultrasound-assisted solvent extraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2010, 1217, 1882–1890. [Google Scholar] [CrossRef]
- Lamas, J.P.; Sanchez-Prado, L.; Lores, M.; Garcia-Jares, C.; Llompart, M. Sorbent trapping solid-phase microextraction of fragrance allergens in indoor air. J. Chromatogr. A 2010, 1217, 5307–5316. [Google Scholar] [CrossRef]
- Regueiro, J.; Garcia-Jares, C.; Llompart, M.; Lamas, J.P.; Cela, R. Development of a method based on sorbent trapping followed by solid-phase microextraction for the determination of synthetic musks in indoor air. J. Chromatogr. A 2009, 1216, 2805–2815. [Google Scholar] [CrossRef]
- Ramírez, N.; Marcé, R.M.; Borrull, F. Development of a thermal desorption-gas chromatography-mass spectrometry method for determining personal care products in air. J. Chromatogr. A 2010, 1217, 4430–4438. [Google Scholar] [CrossRef]
- Balci, E.; Genisoglu, M.; Sofuoglu, S.C.; Sofuoglu, A. Indoor air partitioning of Synthetic Musk Compounds: Gas, particulate matter, house dust, and window film. Sci. Total Environ. 2020, 729, 138798. [Google Scholar] [CrossRef] [PubMed]
- Fontal, M.; van Drooge, B.L.; Grimalt, J.O. A rapid method for the analysis of methyl dihydrojasmonate and galaxolide in indoor and outdoor air particulate matter. J. Chromatogr. A 2016, 1447, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Van Drooge, B.L.; Rivas, I.; Querol, X.; Sunyer, J. Organic Air Quality Markers of Indoor and Outdoor PM 2. 5 Aerosols in Primary Schools from Barcelona. Int. J. Environ. Res. Public Health 2020, 17, 3685. [Google Scholar] [CrossRef] [PubMed]
- Kruza, M.; McFiggans, G.; Waring, M.S.; Wells, J.R.; Carslaw, N. Indoor secondary organic aerosols: Towards an improved representation of their formation and composition in models. Atmos. Environ. 2020, 240, 117784. [Google Scholar] [CrossRef]
- Angulo-Milhem, S.; Verriele, M.; Nicolas, M.; Thevenet, F. Indoor use of essential oils: Emission rates, exposure time and impact on air quality. Atmos. Environ. 2021, 244, 117863. [Google Scholar] [CrossRef]
- Bokowa, A.; Diaz, C.; Koziel, J.A.; Mcginley, M.; Barclay, J.; Guillot, J.; Sneath, R.; Capelli, L.; Zorich, V. Summary and Evaluation of the Odour Regulations Worldwide. Atmosphere 2021, 12, 206. [Google Scholar] [CrossRef]
- Delgado-Saborit, J.M.; Aquilina, N.; Baker, S.; Harrad, S.; Meddings, C.; Harrison, R.M. Determination of atmospheric particulate-phase polycyclic aromatic hydrocarbons from low volume air samples. Anal. Methods 2010, 2, 231–242. [Google Scholar] [CrossRef] [Green Version]
- Hayward, S.J.; Lei, Y.D.; Wania, F. Sorption of a diverse set of organic chemical vapors onto XAD-2 resin: Measurement, prediction and implications for air sampling. Atmos. Environ. 2011, 45, 296–302. [Google Scholar] [CrossRef]
- Balducci, C.; Cecinato, A.; Paolini, V.; Guerriero, E.; Perilli, M.; Romagnoli, P.; Tortorella, C.; Nacci, R.M.; Giove, A.; Febo, A. Volatilization and oxidative artifacts of PM bound PAHs at low volume sampling (2): Evaluation and comparison of mitigation strategies effects. Chemosphere 2017, 189, 330–339. [Google Scholar] [CrossRef]
- Cerasa, M.; Guerriero, E.; Mosca, S. Evaluation of Extraction Procedure of PCDD/Fs, PCBs and Chlorobenzenes from Activated Carbon Fibers (ACFs). Molecules 2021, 26, 6407. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Carvone (Mentha spicata L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780124166448. [Google Scholar]
- Mercer, D.G.; Rodriguez-Amaya, D.B. Reactions and interactions of some food additives. In Chemical Changes During Processing and Storage of Foods; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128173800. [Google Scholar]
- Kholibrina, C.R.; Aswandi, A. The Consumer Preferences for New Sumatran Camphor Essential Oil-based Products using a Conjoint Analysis Approach. IOP Conf. Ser. Earth Environ. Sci. 2021, 715, 012078. [Google Scholar] [CrossRef]
- Cansian, R.L.; Astolfi, V.; Cardoso, R.I.; Paroul, N.; Roman, S.S.; Mielniczki-Pereira, A.A.; Pauletti, G.F.; Mossi, A.J. Atividade inseticida e repelente do óleo essencial de Cinnamomum camphora var. linaloolifera Y. Fujita (Ho-Sho) e Cinnamomum camphora (L.) J Presl. var. hosyo (Hon-Sho) sobre Sitophilus zeamais Mots. (Coleoptera, Curculionedae). Rev. Bras. Plantas Med. 2015, 17, 769–773. [Google Scholar] [CrossRef]
- Zuccarini, P.; Soldani, G. Camphor: Benefits and risks of a widely used natural product. Acta Biol. Szeged. 2009, 53, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Cobo-Golpe, M.; Ramil, M.; Cela, R.; Rodríguez, I. Portable dehumidifiers condensed water: A novel matrix for the screening of semi-volatile compounds in indoor air. Chemosphere 2020, 251, 126346. [Google Scholar] [CrossRef] [PubMed]



| Compound | MW | CAS | Retention Time (min) | Quantitative (m/z) | Confirmation (m/z) |
|---|---|---|---|---|---|
| Salicylaldehyde | 122.12 | 90-02-8 | 6.28 | 122 | 65, 121 |
| Camphor | 152.23 | 464-49-1 | 8.88 | 81 | 95, 108 |
| Folione | 154.21 | 111-12-6 | 10.42 | 123 | 95, 111 |
| Neral | 152.23 | 5392-40-5 | 11.63 | 119 | 69, 84 |
| Carvone | 150.22 | 2244-16-8 | 11.74 | 108 | 54, 82 |
| Geranial | 152.23 | 141-27-5 | 12.51 | 152 | 84, 83 |
| DMBCA | 192.25 | 151-05-1 | 14.06 | 132 | 91, 117 |
| Geranyl acetate | 196.29 | 105-87-1 | 16.00 | 136 | 68, 93 |
| β-Damascenone | 190.28 | 23696-85-7 | 16.10 | 175 | 69, 190 |
| δ-Damascone | 192.30 | 57378-68-4 | 16.38 | 123 | 69, 192 |
| β-Damascone | 192.30 | 23726-91-2 | 17.02 | 177 | 123, 192 |
| Coumarin | 229.16 | 91-64-5 | 17.66 | 146 | 89, 118 |
| α-Isomethylionone | 206.32 | 127-51-5 | 18.98 | 150 | 135, 206 |
| Eugenyl acetate | 206.24 | 93-28-7 | 20.26 | 164 | 131, 149 |
| 3-Propylidenephthalide | 174.2 | 17369-59-4 | 21.50 | 159 | 104, 174 |
| α-Amylcinnamaldehyde | 202.29 | 78605-96-6 | 23.41 | 129 | 201, 202 |
| ISO E® γ | 234.38 | 68155-67-9 | 23.65 | 191 | 109, 121 |
| Musk xylene | 297.26 | 81-15-2 | 28.33 | 282 | 127, 297 |
| Musk ketone | 294.30 | 81-14-1 | 30.77 | 279 | 280, 294 |
| Benzyl cinnamate | 238.28 | 103-41-3 | 32.76 | 131 | 192, 193 |
| Naphthalene-d8 | 136.22 | 1146-65-2 | 9.91 | 108 | 136 |
| 2-Methylnaphthalene-d10 | 152.26 | 7297-45-2 | 13.11 | 122 | 152 |
| Diethyl phthalate-d4 | 226.26 | 93952-12-6 | 22.04 | 181 | 153 |
| Pyrene-d10 | 212.31 | 7297-45-2 | 33.06 | 106 | 212 |
| Benzo[a]anthracene-d12 | 240.36 | 1718-53-2 | 30.08 | 120 | 240 |
| Compound | Calibration Curve | R | LODinst |
|---|---|---|---|
| Salicylaldehyde | Y = −0.0153816 + 1.12523 X | 0.9959 | 0.001 |
| Camphor | Y = −0.00116375 + 1.10579 X | 0.9997 | 0.001 |
| Folione | Y = −0.0066146 + 0.477204 X | 0.9997 | 0.004 |
| Neral | Y = −0.00723682 + 0.355397 X | 0.9998 | 0.006 |
| Carvone | Y = −0.0138041 + 0.775145 X | 0.9997 | 0.003 |
| Geranial | Y = −0.00914425 + 0.353934 X | 0.9994 | 0.007 |
| DMBCA | Y = −0.0171582 + 1.34708 X | 0.9998 | 0.001 |
| Geranyl acetate | Y = −0.00736214 + 0.312877 X | 0.9997 | 0.012 |
| β-Damascenone | Y = −0.0567008 + 2.59694 X | 0.9995 | 0.001 |
| δ-Damascone | Y = −0.0204197 + 0.950358 X | 0.9993 | 0.004 |
| β-Damascone | Y = −0.0483275 + 2.02308 X | 0.9993 | 0.002 |
| Coumarin | Y = −0.027222 + 1.94787 X | 0.9997 | 0.002 |
| α-Isomethylionone | Y = −0.0390646 + 1.8334 X | 0.9994 | 0.002 |
| Eugenyl acetate | Y = −0.0778768 + 3.53241 X | 0.9996 | 0.001 |
| 3-Propylidenephthalide | Y = −0.0430587 + 2.29359 X | 0.9996 | 0.001 |
| α-Amylcinnamaldehyde | Y = −0.0496146 + 1.74195 X | 0.9992 | 0.002 |
| ISO E® γ | Y = −0.00185945 + 0.122102 X | 0.9985 | 0.026 |
| Musk xylene | Y = −0.0189596 + 0.656847 X | 0.9988 | 0.005 |
| Musk ketone | Y = −0.0329199 + 0.791557 X | 0.9967 | 0.005 |
| Benzyl cinnamate | Y = −0.0813413 + 1.95804 X | 0.9954 | 0.002 |
| Compound | Level 1 (100 ng) | Level 2 (3000 ng) | Level 3 (7500 ng) |
|---|---|---|---|
| %R (SD) | %R (SD) | %R (SD) | |
| Salicylaldehyde | 77.1 (8) | 92.7 (7) | 83.4 (14) |
| Camphor | 82.7 (7) | 77.9 (1) | 86.9 (8) |
| Folione | 72.8 (4) | 88.7 (3) | 97.7 (0) |
| Neral | 71.4 (7) | 86.9 (4) | 92.2 (1) |
| Carvone | 64.6 (9) | 75.7 (3) | 93.7 (3) |
| Geranial | 66.7 (6) | 85.1 (7) | 91.9 (4) |
| DMBCA | 74.8 (2) | 82 (6) | 92.5 (5) |
| Geranyl acetate | 66.9 (1) | 70.4 (5) | 84.3 (3) |
| ß-Damascenone | 69.3 (1) | 66.2 (5) | 86.2 (6) |
| δ-Damascone | 70.4 (0) | 68.7 (4) | 86.3 (6) |
| ß-Damascone | 72.4 (1) | 73.0 (5) | 91.3 (6) |
| Coumarin | 67.1 (5) | 70.7 (5) | 86.4 (4) |
| a-Isomethylionone | 79.6 (1) | 70.5 (4) | 89.8 (6) |
| Eugenyl acetate | 75.4 (1) | 81.2 (4) | 88.9 (5) |
| 3-Propylidenephthalide | 69.1 (0) | 77.9 (2) | 83.3 (3) |
| a-Amylcinnamaldehyde | 70.9 (1) | 85.1 (3) | 91.4 (4) |
| ISO E® γ | 78.3 (3) | 83.1 (4) | 92.2 (3) |
| Musk xylene | 90 (5) | 90.5 (11) | 92.4 (6) |
| Musk ketone | 65.4 (1) | 76.9 (5) | 73.6 (3) |
| Benzyl cinnamate | 79.3 (5) | 88.1 (5) | 115 (8) |
| Compound | House | Coffee Bar | Compound | House | Coffee Bar |
|---|---|---|---|---|---|
| (ng m−3) | (ng m−3) | (ng m−3) | (ng m−3) | ||
| Salicylaldehyde | 54.4 ± 4.3 | 32 ± 2.6 | ß-Damascone | ND 1 | 0.14 ± 0.01 |
| Camphor | 157 ± 11.0 | 75.7 ± 5.3 | Coumarin | 29.6 ± 1.5 | 39.1 ± 1.9 |
| Folione | ND | ND | α-Isomethylionone | 63.2 ± 0.6 | 74.1 ± 0.7 |
| Neral | 46.8 ± 1.8 | 162 ± 6.5 | Eugenyl acetate | 0.45 ± 0.01 | 0.86 ± 0.01 |
| Carvone | 39.5 ± 3.2 | 349 ± 6.5 | 3-Propylidenephthalide | 0.68 ± 0.01 | 1.04 ± 0.01 |
| Geranial | 39.8 ± 2.4 | 19.4 ± 1.2 | a-Amylcinnamaldehyde | 8.33 ± 0.08 | 19.1 ± 0.2 |
| DMBCA | 17.2 ± 0.3 | 17.1 ± 0.3 | ISO E® γ | ND | ND |
| Geranyl acetate | 18.1 ± 0.2 | 17.2 ± 0.2 | Benzyl cinnamate | 0.97 ± 0.04 | 0.96 ± 0.04 |
| ß-Damascenone | 7.85 ± 0.01 | ND | Musk xylene | 2.38 ± 0.02 | 1.32 ± 0.01 |
| δ-Damascone | ND | 1.03 ± 0.07 | Musk ketone | 1.25 ± 0.06 | 1.54 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balducci, C.; Cerasa, M.; Avino, P.; Ceci, P.; Bacaloni, A.; Garofalo, M. Analytical Determination of Allergenic Fragrances in Indoor Air. Separations 2022, 9, 99. https://0-doi-org.brum.beds.ac.uk/10.3390/separations9040099
Balducci C, Cerasa M, Avino P, Ceci P, Bacaloni A, Garofalo M. Analytical Determination of Allergenic Fragrances in Indoor Air. Separations. 2022; 9(4):99. https://0-doi-org.brum.beds.ac.uk/10.3390/separations9040099
Chicago/Turabian StyleBalducci, Catia, Marina Cerasa, Pasquale Avino, Paolo Ceci, Alessandro Bacaloni, and Martina Garofalo. 2022. "Analytical Determination of Allergenic Fragrances in Indoor Air" Separations 9, no. 4: 99. https://0-doi-org.brum.beds.ac.uk/10.3390/separations9040099