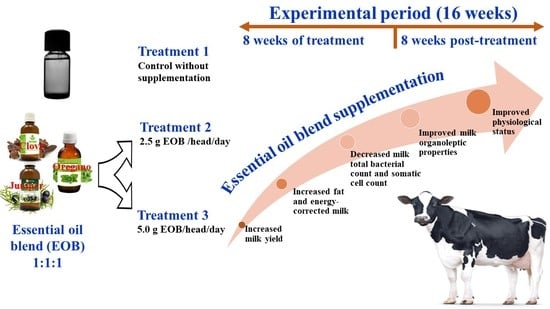
Effect of an Essential Oil Blend on Dairy Cow Performance during Treatment and Post-Treatment Periods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Management
2.2. Ration and Feeding Regime
2.3. Milk Yield and Composition
2.4. Milk Total Bacterial Acount
2.5. Milk Somatic Cell Count
2.6. Hematological and Serum Biochemical Parameters
2.7. Statistical Analyses
3. Results
3.1. Milk Yield and Feed Efficiency
3.2. Milk Composition
3.3. Milk Total Bacterial and Somatic Cell Counts
3.4. Hematology and Serum Biochemical Evaluation
4. Discussion
4.1. Dry Matter Intake and Feed Efficiency
4.2. Milk Yield and Composition
4.3. Milk Total Bacterial Count and Somatic Cell Count
4.4. Hematological Parameters and Blood Metabolites
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martinez, S.; Madrid, J.; Hernandez, F.; Megias, M.; Sotomayor, J.; Jordan, M. Effect of thyme essential oils (Thymus hyemalis and Thymus zygis) and monensin on in vitro ruminal degradation and volatile fatty acid production. J. Agric. Food Chem. 2006, 54, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, K.; Paramasivam, M.; Sasikala, M.; Tamilam, T.; Sumithra, A. Review on antibiotic residues in animal products and its impact on environments and human health. J. Entomol. Zool. Stud. 2017, 5, 1446–1451. [Google Scholar]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Nagpal, R.; Shrivastava, B.; Kumar, N.; Dhewa, T.; Sahay, H. Microbial Feed Additives. In Rumen Microbiology: From Evolution to Revolution; Springer: New Delhi, India, 2015; pp. 161–175. [Google Scholar]
- Carro, M.; Ungerfeld, E. Utilization of Organic Acids to Manipulate Ruminal Fermentation and Improve Ruminant Productivity. In Rumen Microbiology: From Evolution to Revolution; Springer: New Delhi, India, 2015; pp. 177–197. [Google Scholar]
- Sallam, S.M.A.; Allam, A.M.; Najadi, S.A. Comparison of two products of direct-fed microbial supplementation on the nutrient utilization and ruminal fermentation in sheep. J. Agric. Sci. 2014, 6, 159. [Google Scholar] [CrossRef] [Green Version]
- Morsy, A.S.; Abdalla, A.L.; Soltan, Y.A.; Sallam, S.M.; El-Azrak, K.E.D.M.; Louvandini, H.; Alencar, S.M. Effect of Brazilian red propolis administration on hematological, biochemical variables and parasitic response of Santa Inês ewes during and after flushing period. Trop. Anim. Health Prod. 2013, 45, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Sallam, S.M.; Bueno, I.C.; Nasser, M.E.; Abdalla, A.L. Effect of eucalyptus (Eucalyptus citriodora) fresh or residue leaves on methane emission in vitro. Ital. J. Anim. Sci. 2010, 9, e58. [Google Scholar]
- Deyno, S.; Mtewa, A.G.; Abebe, A.; Hymete, A.; Makonnen, E.; Bazira, J.; Alele, P.E. Essential oils as topical anti-infective agents: A systematic review and meta-analysis. Complement. Ther. Med. 2019, 47, 102224. [Google Scholar] [CrossRef]
- Herago, T.; Agonafir, A. Growth promoters in cattle. Adv. Biol. Res. 2017, 11, 24–34. [Google Scholar]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.; Castillejos, L.; Ferret, A. Invited review: Essential oils as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef] [Green Version]
- Greathead, H. Plants and plant extracts for improving animal productivity. Proc. Nutr. Soc. 2003, 62, 279–290. [Google Scholar] [CrossRef]
- Aali, E.; Mahmoudi, R.; Kazeminia, M.; Hazrati, R.; Azarpey, F. Essential oils as natural medicinal substances. Tehran Univ. Med. J. 2017, 75, 480–489. [Google Scholar]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.G.; Liu, T.; Hu, Q.P.; Cao, X.M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef] [PubMed]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef]
- Selles, S.M.A.; Kouidri, M.; Belhamiti, B.T.; Amrane, A.A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. J. Food Meas. Charact. 2020, 14, 2352–2358. [Google Scholar] [CrossRef]
- Dutra, T.V.; Castro, J.C.; Menezes, J.L.; Ramos, T.R.; do Prado, I.N.; Junior, M.M.; Mikcha, J.M.G.; de Abreu Filho, B.A. Bioactivity of oregano (Origanum vulgare) essential oil against Alicyclobacillus spp. Ind. Crops Prod. 2019, 129, 345–349. [Google Scholar] [CrossRef]
- Olijhoek, D.; Hellwing, A.L.F.; Grevsen, K.; Haveman, L.; Chowdhury, M.R.; Løvendahl, P.; Weisbjerg, M.R.; Noel, S.J.; Højberg, O.; Wiking, L. Effect of dried oregano (Origanum vulgare L.) plant material in feed on methane production, rumen fermentation, nutrient digestibility, and milk fatty acid composition in dairy cows. J. Dairy Sci. 2019, 102, 9902–9918. [Google Scholar] [CrossRef] [PubMed]
- Roofchaee, A.; Irani, M.; Ebrahimzadeh, M.A.; Akbari, M.R. Effect of dietary oregano (Origanum vulgare L.) essential oil on growth performance, cecal microflora and serum antioxidant activity of broiler chickens. Afr. J. Biotechnol. 2011, 10, 6177–6183. [Google Scholar]
- Davidson, P.M.; Naidu, A.S. Phyto-phenols. In Natural Food Antimicrobial Systems; Naidu, A.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 265–294. [Google Scholar]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Schmidt, E.; Wanner, J.; Jirovetz, L.; Trifonova, D.; Krastev, L.; Krastanov, A. Chemical composition and antioxidant properties of juniper berry (Juniperus communis L.) essential oil. Action of the essential oil on the antioxidant protection of Saccharomyces cerevisiae model organism. Antioxidants 2014, 3, 81–98. [Google Scholar] [CrossRef] [Green Version]
- Orav, A.; Kailas, T.; Müürisepp, M. Chemical investigation of the essential oil from berries and needles of common juniper (Juniperus communis L.) growing wild in Estonia. Nat. Prod. Res. 2010, 24, 1789–1799. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Semerdjieva, I.B.; Dincheva, I.; Kacaniova, M.; Astatkie, T.; Radoukova, T.; Schlegel, V. Antimicrobial and antioxidant activity of Juniper galbuli essential oil constituents eluted at different times. Ind. Crops Prod. 2017, 109, 529–537. [Google Scholar] [CrossRef]
- Radoukova, T.; Zheljazkov, V.; Semerdjieva, I.; Dincheva, I.; Stoyanova, A.; Kačániová, M.; Marković, T.; Radanović, D.; Astatkie, T.; Salamon, I. Differences in essential oil yield, composition, and bioactivity of three juniper species from Eastern Europe. Ind. Crops Prod. 2018, 124, 643–652. [Google Scholar] [CrossRef]
- Benchaar, C.; Hristov, A.N.; Greathead, H. Essential Oils as Feed Additives in Ruminant Nutrition. In Phytogenics in Animal Nutrition; Steiner, T., Ed.; Nottingham University Press: Nottingham, UK, 2009; pp. 111–146. [Google Scholar]
- Blanch, M.; Carro, M.; Ranilla, M.J.; Viso, A.; Vázquez-Añón, M.; Bach, A. Influence of a mixture of cinnamaldehyde and garlic oil on rumen fermentation, feeding behavior and performance of lactating dairy cows. Anim. Feed Sci. Technol. 2016, 219, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Suksombat, W.; Nanon, A.; Meeprom, C.; Lounglawan, P. Feed degradability, rumen fermentation and blood metabolites in response to essential oil addition to fistulated non-lactating dairy cow diets. Anim. Sci. J. 2017, 88, 1346–1351. [Google Scholar] [CrossRef]
- Elcoso, G.; Zweifel, B.; Bach, A. Effects of a blend of essential oils on milk yield and feed efficiency of lactating dairy cows. Appl. Anim. Sci. 2019, 35, 304–311. [Google Scholar] [CrossRef]
- Braun, H.S.; Schrapers, K.T.; Mahlkow-Nerge, K.; Stumpff, F.; Rosendahl, J. Dietary supplementation of essential oils in dairy cows: Evidence for stimulatory effects on nutrient absorption. Animal 2019, 13, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Spanghero, M.; Zanfi, C.; Fabbro, E.; Scicutella, N.; Camellini, C. Effects of a blend of essential oils on some end products of in vitro rumen fermentation. Anim. Feed Sci. Technol. 2008, 145, 364–374. [Google Scholar] [CrossRef]
- Castillejos, L.; Calsamiglia, S.; Ferret, A.; Losa, R. Effects of a specific blend of essential oil compounds and the type of diet on rumen microbial fermentation and nutrient flow from a continuous culture system. Anim. Feed Sci. Technol. 2005, 119, 29–41. [Google Scholar] [CrossRef]
- Castillejos, L.; Calsamiglia, S.; Ferret, A.; Losa, R. Effects of dose and adaptation time of a specific blend of essential oil compounds on rumen fermentation. Anim. Feed Sci. Technol. 2007, 132, 186–201. [Google Scholar] [CrossRef]
- Kung, L.; Williams, P.; Schmidt, R.; Hu, W. A blend of essential plant oils used as an additive to alter silage fermentation or used as a feed additive for lactating dairy cows. J. Dairy Sci. 2008, 91, 4793–4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassoul, M.; Shaver, R. Effect of a mixture of supplemental dietary plant essential oils on performance of periparturient and early lactation dairy cows. J. Dairy Sci. 2009, 92, 1734–1740. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.G.; El-Zarkouny, S.Z.; El-Shazly, K.A.; Sallam, S.M.A. Impact of essential oils blend on methane emission, rumen fermentation characteristics and nutrient digestibility in Barki sheep. J. Agric. Sci. 2014, 6, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Malekkhahi, M.; Tahmasbi, A.M.; Naserian, A.A.; Danesh Mesgaran, M.; Kleen, J.; Parand, A. Effects of essential oils, yeast culture and malate on rumen fermentation, blood metabolites, growth performance and nutrient digestibility of Baluchi lambs fed high-concentrate diets. J. Anim. Physiol. Anim. Nutr. 2015, 99, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Lettat, A.; Hassanat, F.; Yang, W.; Forster, R.; Petit, H.; Chouinard, P. Eugenol for dairy cows fed low or high concentrate diets: Effects on digestion, ruminal fermentation characteristics, rumen microbial populations and milk fatty acid profile. Anim. Feed Sci. Technol. 2012, 178, 139–150. [Google Scholar] [CrossRef]
- Santos, M.; Robinson, P.; Williams, P.; Losa, R. Effects of addition of an essential oil complex to the diet of lactating dairy cows on whole tract digestion of nutrients and productive performance. Anim. Feed Sci. Technol. 2010, 157, 64–71. [Google Scholar] [CrossRef]
- Giannenas, I.; Skoufos, J.; Giannakopoulos, C.; Wiemann, M.; Gortzi, O.; Lalas, S.; Kyriazakis, I. Effects of essential oils on milk production, milk composition, and rumen microbiota in Chios dairy ewes. J. Dairy Sci. 2011, 94, 5569–5577. [Google Scholar] [CrossRef] [PubMed]
- Morshedy, S.A. Effect of Blending Volatile Oil Mixtures on Rumen Fermentation, Methane Emission and Milk Production in Ruminants; University of Alexandria: Alexandria, Egypt, 2015. [Google Scholar]
- Yang, W.; Benchaar, C.; Ametaj, B.; Chaves, A.; He, M.; McAllister, T. Effects of garlic and juniper berry essential oils on ruminal fermentation and on the site and extent of digestion in lactating cows. J. Dairy Sci. 2007, 90, 5671–5681. [Google Scholar] [CrossRef] [Green Version]
- Benchaar, C.; Petit, H.; Berthiaume, R.; Whyte, T.; Chouinard, P. Effects of addition of essential oils and monensin premix on digestion, ruminal fermentation, milk production, and milk composition in dairy cows. J. Dairy Sci. 2006, 89, 4352–4364. [Google Scholar] [CrossRef]
- NRC. National Research Council Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- AOAC. Association of Official Analytical Chemists Official Method of Analysis; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Van Soest, P.J.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Van Soest, P.J. Collaborative study of acid-detergent fiber and lignin. J. Assoc. Off. Anal. Chem. 1973, 56, 781–784. [Google Scholar] [CrossRef]
- Gaines, W.L.; Davidson, F.A. Relation between Percentage Fat Content and Yield of Milk: Correction of Milk Yield for Fat Content; Bulletin No. 245; University of Illinois, Agricultural Experiment Station, Urbana-Champaign Campus: Champaign, IL, USA, 1923. [Google Scholar]
- Tyrrell, H.; Reid, J. Prediction of the energy value of cow’s milk 1, 2. J. Dairy Sci. 1965, 48, 1215–1223. [Google Scholar] [CrossRef]
- Houghtby, G.; Maturin, L.; Koenig, E. Microbiological Count Methods. In Standard Methods for the Examination of Dairy Products; Marshal, T.R., Ed.; American Public Health Association: Washington, DC, USA, 1992; pp. 213–246. [Google Scholar]
- SAS Institute Inc. SAS/STAT User’s Guide: Version 9, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Duncan, D.B. Multiple range and F tests. Biometric 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Sallam, S.; Bueno, I.; Brigide, P.; Godoy, P.; Vitti, D.; Abdalla, A. Investigation of potential new opportunities for plant extracts on rumen microbial fermentation in vitro. Nutr. Foraging Ecol. Sheep Goats 2009, 303, 255–260. [Google Scholar]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Effects of alfalfa extract, anise, capsicum, and a mixture of cinnamaldehyde and eugenol on ruminal fermentation and protein degradation in beef heifers fed a high-concentrate diet. J. Anim. Sci. 2006, 84, 2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benchaar, C.; Petit, H.; Berthiaume, R.; Ouellet, D.; Chiquette, J.; Chouinard, P. Effects of essential oils on digestion, ruminal fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed alfalfa silage or corn silage. J. Dairy Sci. 2007, 90, 886–897. [Google Scholar] [CrossRef] [Green Version]
- Malecky, M.; Broudiscou, L.; Schmidely, P. Effects of two levels of monoterpene blend on rumen fermentation, terpene and nutrient flows in the duodenum and milk production in dairy goats. Anim. Feed Sci. Technol. 2009, 154, 24–35. [Google Scholar] [CrossRef]
- Chaves, A.V.; Stanford, K.; Dugan, M.E.R.; Gibson, L.L.; McAllister, T.A.; Van Herk, F.; Benchaar, C. Effects of cinnamaldehyde, garlic and juniper berry essential oils on rumen fermentation, blood metabolites, growth performance, and carcass characteristics of growing lambs. Livest. Sci. 2008, 117, 215–224. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Zhou, H. Influences of flavomycin, ropadiar, and saponin on nutrient digestibility, rumen fermentation, and methane emission from sheep. Anim. Feed Sci. Technol. 2009, 148, 157–166. [Google Scholar] [CrossRef]
- Morsy, T.; Kholif, S.; Matloup, O.; Abdo, M.; El-Shafie, M. Impact of anise, clove and juniper oils as feed additives on the productive performance of lactating goats. Int. J. Dairy Sci. 2012, 7, 20–28. [Google Scholar]
- Geraci, J.I.; Garciarena, A.D.; Gagliostro, G.A.; Beauchemin, K.A.; Colombatto, D. Plant extracts containing cinnamaldehyde, eugenol and capsicum oleoresin added to feedlot cattle diets: Ruminal environment, short term intake pattern and animal performance. Anim. Feed Sci. Technol. 2012, 176, 123–130. [Google Scholar] [CrossRef]
- Da Silva, R.B.; Pereira, M.N.; de Araujo, R.C.; Silva, W.d.R.; Pereira, R.A.N. A blend of essential oils improved feed efficiency and affected ruminal and systemic variables of dairy cows. Transl. Anim. Sci. 2020, 4, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Azrak, K.M.K. Effect of Essential Oils Administration on Some Productive and Reproductive Traits in Sheep; University of Alexandria: Alexandria, Egypt, 2012. [Google Scholar]
- De Jesus, E.F.; Del Valle, T.; Calomeni, G.; Silva, T.; Takiya, C.; Vendramini, T.; Paiva, P.; Silva, G.; Netto, A.; Rennó, F. Influence of a blend of functional oils or monensin on nutrient intake and digestibility, ruminal fermentation and milk production of dairy cows. Anim. Feed Sci. Technol. 2016, 219, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Kırkpınar, F.; Ünlü, H.B.; Özdemir, G. Effects of oregano and garlic essential oils on performance, carcase, organ and blood characteristics and intestinal microflora of broilers. Livest. Sci. 2011, 137, 219–225. [Google Scholar] [CrossRef]
- Svennersten-Sjaunja, K.; Olsson, K. Endocrinology of milk production. Domest. Anim. Endocrinol. 2005, 29, 241–258. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [Green Version]
- Harding, F. World Milk Production. In Milk Quality; Springer: Boston, MA, USA, 1995; pp. 1–2. [Google Scholar]
- Heinrichs, J.; Jones, C.; Bailey, K. Milk components: Understanding the Causes and Importance of Milk Fat and Protein Variation in Your Dairy Herd. In Dairy and Animal Science Fact Sheet; Department of Dairy and Animal Science, The Pennsylvania State University: State College, PA, USA, 1997; Volume 5, pp. 1e–8e. [Google Scholar]
- Gaunt, S. Genetic variation in the yields and contents of milk constituents (quantitative differences; milk proteins, lactose, ash, non-fat solids; dairy cows breeds; Ayrshire; Brown Swiss; Guernsey; Holstein Friesian; Jersey). Bull. Int. Dairy Fed. Belgium 1980, 125, 73–82. [Google Scholar]
- Flores, A.J.; Garciarena, A.D.; Vieyra, J.M.H.; Beauchemin, K.A.; Colombatto, D. Effects of specific essential oil compounds on the ruminal environment, milk production and milk composition of lactating dairy cows at pasture. Anim. Feed Sci. Technol. 2013, 186, 20–26. [Google Scholar] [CrossRef]
- Plaizier, J.; Khafipour, E.; Li, S.; Gozho, G.; Krause, D. Subacute ruminal acidosis (SARA), endotoxins and health consequences. Anim. Feed Sci. Technol. 2012, 172, 9–21. [Google Scholar] [CrossRef]
- Tolle, A. The Microflora of the Udder. In Factors Influencing the Bacteriological Quality of Raw Milk; Bulletin International Dairy Federation: Brussels, Belgium, 1980; p. 4. [Google Scholar]
- Bramley, A.; McKinnon, C. The Microbiology of Raw Milk. In Dairy Microbiology the Microbiology of Milk, 2nd ed.; Robinson, R.K., Ed.; Elsevier Science: London, UK, 1990; Volume 1, pp. 163–208. [Google Scholar]
- Barrett, D. High somatic cell counts-a persistent problem. Ir. Vet. J. 2002, 55, 173. [Google Scholar]
- Juozaitiene, V.; Juozaitis, A.; Micikeviciene, R. Relationship between somatic cell count and milk production or morphological traits of udder in Black-and-White cows. Turk. J. Vet. Anim. Sci. 2006, 30, 47–51. [Google Scholar]
- Cinar, M.; Serbester, U.; Ceyhan, A.; Gorgulu, M. Effect of somatic cell count on milk yield and composition of first and second lactation dairy cows. Ital. J. Anim. Sci. 2015, 14, 3646. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Morsy, N.F.S. Chemical structure, quality indices and bioactivity of essential oil constituents. Act. Ingred. Aromat. Med. Plants 2017, 175–206. [Google Scholar]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragrance J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ametaj, B.; Benchaar, C.; He, M.; Beauchemin, K. Cinnamaldehyde in feedlot cattle diets: Intake, growth performance, carcass characteristics, and blood metabolites. J. Anim. Sci. 2010, 88, 1082–1092. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ametaj, B.; Benchaar, C.; Beauchemin, K. Dose response to cinnamaldehyde supplementation in growing beef heifers: Ruminal and intestinal digestion. J. Anim. Sci. 2010, 88, 680–688. [Google Scholar] [CrossRef]
- Anassori, E.; Dalir-Naghadeh, B.; Pirmohammadi, R.; Hadian, M. Changes in blood profile in sheep receiving raw garlic, garlic oil or monensin. J. Anim. Physiol. Anim. Nutr. 2015, 99, 114–122. [Google Scholar] [CrossRef]
- Serbester, U.; Çinar, M.; Ceyhan, A.; Erdem, H.; Görgülü, M.; Kutlu, H.; Çelik, L.; Yücelt, Ö.; Cardoza, P. Effect of essential oil combination on performance, milk composition, blood parameters and pregnancy rate in early lactating dairy cows during heat exposure. J. Anim. Plant Sci. 2012, 22, 556–563. [Google Scholar]
- Kowalik, B.; Skomial, J.; Pajak, J.J.; Taciak, M.; Majewska, M.; Belzecki, G. Population of ciliates, rumen fermentation indicators and biochemical parameters of blood serum in heifers fed diets supplemented with yeast(Saccharomyces cerevisiae) preparation. Anim. Sci. Pap. Rep. 2012, 30, 329–338. [Google Scholar]
- Chaves, A.; Dugan, M.; Stanford, K.; Gibson, L.; Bystrom, J.; McAllister, T.; Van Herk, F.; Benchaar, C. A dose-response of cinnamaldehyde supplementation on intake, ruminal fermentation, blood metabolites, growth performance, and carcass characteristics of growing lambs. Livest. Sci. 2011, 141, 213–220. [Google Scholar] [CrossRef]
- Castillo, C.; Benedito, J.L.; Vázquez, P.; Pereira, V.; Méndez, J.; Sotillo, J.; Hernández, J. Effects of supplementation with plant extract product containing carvacrol, cinnamaldehyde and capsaicin on serum metabolites and enzymes during the finishing phase of feedlot-fed bull calves. Anim. Feed Sci. Technol. 2012, 171, 246–250. [Google Scholar] [CrossRef]

| Ingredients of Concentrate Mixture | (g/kg of DM Basis) | |
|---|---|---|
| Ground yellow corn | 330 | |
| Wheat bran | 300 | |
| Cottonseed meal | 280 | |
| Soybean meal | 50 | |
| Calcium carbonate | 20 | |
| Sodium chloride | 10 | |
| Di-calcium phosphate | 7 | |
| Trace minerals 1 | 3 | |
| Chemical Composition (g/kg of DM Basis) | Concentrate Mixture (g/kg of DM Basis) | Egyptian Clover (g/kg of DM Basis) |
| Dry matter | 900.6 | 157.7 |
| Organic matter | 961.4 | 857.0 |
| Ash | 38.6 | 143.0 |
| Crude protein | 178.1 | 169.4 |
| Ether extract | 57.9 | 26.2 |
| Neutral detergent fiber | 601.4 | 619.6 |
| Acid detergent fiber | 167.1 | 364.8 |
| Lignin (sa) | 47.9 | 103.3 |
| Non-fiber carbohydrates | 124.0 | 41.8 |
| Hemicellulose | 434.3 | 254.8 |
| Cellulose | 119.1 | 260.7 |
| Items | Control | EOB2.5 | EOB5.0 | p Value | |||
|---|---|---|---|---|---|---|---|
| T | W | T × W | |||||
| DMI (kg/day) | Treatment | 20.71 ± 0.03 a | 20.51 ± 0.04 b | 20.50 ± 0.04 b | 0.001 | 0.001 | 0.81 |
| Post-treatment | 20.65 ± 0.08 | 20.76 ± 0.07 | 20.69 ± 0.08 | 0.46 | 0.001 | 0.01 | |
| MY (kg/day) | Treatment | 25.76 ± 0.31 b | 26.84 ± 0.22 a | 26.17 ± 0.29 ab | 0.02 | 0.001 | 0.82 |
| Post-treatment | 23.94 ± 0.42 | 24.99 ± 0.42 | 24.71 ± 0.44 | 0.20 | 0.24 | 0.64 | |
| FCM (kg/day) | Treatment | 24.25 ± 0.52 | 25.29 ± 0.22 | 24.87 ± 0.28 | 0.14 | 0.30 | 0.35 |
| Post-treatment | 22.48 ± 0.49 | 23.95 ± 0.53 | 23.53 ± 0.64 | 0.18 | 0.64 | 0.81 | |
| ECM (kg/day) | Treatment | 25.74 ± 0.51 | 26.81 ± 0.37 | 26.32 ± 0.40 | 0.21 | 0.32 | 0.44 |
| Post-treatment | 23.91 ± 0.55 | 25.27 ± 0.58 | 24.93 ± 0.69 | 0.33 | 0.36 | 0.89 | |
| FE (kg milk/kg DMI) | Treatment | 1.24 ± 0.02 b | 1.31 ± 0.01 a | 1.28 ± 0.01 ab | 0.001 | 0.54 | 0.79 |
| Post-treatment | 1.16 ± 0.02 | 1.20 ± 0.02 | 1.19 ± 0.02 | 0.38 | 0.04 | 0.77 | |
| Items | Control | EOB2.5 | EOB5.0 | p Value | |||
|---|---|---|---|---|---|---|---|
| T | W | T × W | |||||
| Fat (%) | Treatment | 3.60 ± 0.06 | 3.62 ± 0.05 | 3.66 ± 0.05 | 0.39 | 0.34 | 0.25 |
| Post-treatment | 3.64 ± 0. 08 | 3.67 ± 0.06 | 3.76 ± 0.08 | 0.53 | 0.91 | 0.99 | |
| Protein (%) | Treatment | 3.05 ± 0.01 b | 3.06 ± 0.01 b | 3.09 ± 0.01 a | 0.03 | 0.001 | 0.70 |
| Post-treatment | 3.06 ± 0.02 | 3.07 ± 0.02 | 3.12 ± 0.03 | 0.16 | 1.00 | 0.97 | |
| Lactose (%) | Treatment | 4.21 ± 0.03 b | 4.22 ± 0.03 ab | 4.28 ± 0.04 a | 0.03 | 0.04 | 0.51 |
| Post-treatment | 4.19 ± 0.04 | 4.22 ± 0.06 | 4.28 ± 0.05 | 0.51 | 0.50 | 0.97 | |
| SNF (%) | Treatment | 7.83 ± 0.05 | 7.87 ± 0.05 | 7.90 ± 0.04 | 0.37 | 0.32 | 0.96 |
| Post-treatment | 7.85 ± 0.08 | 7.91 ± 0.06 | 7.94 ± 0.07 | 0.87 | 0.18 | 0.90 | |
| Ash (%) | Treatment | 0.57 ± 0.01 b | 0.58 ± 0.01 a | 0.60 ± 0.01 a | 0.001 | 0.01 | 0.49 |
| Post-treatment | 0.60 ± 0.01 b | 0.61 ± 0.01 ab | 0.63 ± 0.01 a | 0.02 | 0.97 | 0.96 | |
| Density | Treatment | 27.02 ± 0.14 | 27.15 ± 0.13 | 27.29 ± 0.13 | 0.36 | 0.03 | 0.95 |
| Post-treatment | 27.24 ± 0.18 | 27.32 ± 0.16 | 27.82 ± 0.18 | 0.06 | 0.79 | 0.37 | |
| Items | Control | EOB2.5 | EOB5.0 | p Value | |||
|---|---|---|---|---|---|---|---|
| T | W | T × W | |||||
| RBCs (×106/mm3 blood) | Treatment | 6.49 ± 0.08 | 6.48 ± 0.08 | 6.37 ± 0.08 | 0.52 | 0.71 | 0.82 |
| Post-treatment | 6.63 ± 0.11 | 6.63 ± 0.13 | 6.64 ± 0.10 | 0.11 | 0.04 | 0.08 | |
| WBCs (×103/mm3 blood) | Treatment | 7.66 ± 0.20 | 7.32 ± 0.20 | 7.35 ± 0.17 | 0.39 | 0.05 | 0.00 |
| Post-treatment | 7.32 ± 0.25 | 7.35 ± 0.24 | 7.27 ± 0.26 | 0.43 | 0.91 | 0.03 | |
| Hb value (g/dL blood) | Treatment | 9.42 ± 0.13 | 9.76 ± 0.15 | 9.59 ± 0.13 | 0.66 | 0.13 | 0.52 |
| Post-treatment | 9.97 ± 0.19 | 10.00 ± 0.18 | 9.97 ± 0.21 | 0.47 | 0.72 | 0.86 | |
| Items | Control | EOB2.5 | EOB5.0 | p Value | |||
|---|---|---|---|---|---|---|---|
| T | W | T × W | |||||
| Total protein (g/dL) | Treatment | 7.77 ± 0.12 b | 8.12 ± 0.09 a | 8.11 ± 0.08 a | 0.02 | 0.57 | 0.52 |
| Post-treatment | 7.62 ± 0.11 b | 8.04 ± 0.16 a | 7.67 ± 0.11 b | 0.05 | 0.07 | 0.90 | |
| Albumin (g/dL) | Treatment | 3.60 ± 0.05 | 3.64 ± 0.06 | 3.74 ± 0.05 | 0.16 | 0.48 | 0.51 |
| Post-treatment | 3.73 ± 0.09 a | 3.49 ± 0.07 b | 3.70 ± 0.06 a | 0.05 | 0.10 | 0.57 | |
| Globulin (g/dL) | Treatment | 4.18 ± 0.12 b | 4.48 ± 0.09 a | 4.37 ± 0.09 a | 0.05 | 0.20 | 0.28 |
| Post-treatment | 3.88 ± 0.14 b | 4.55 ± 0.14 a | 3.97 ± 0.14 b | 0.001 | 0.01 | 0.76 | |
| Cholesterol (mg/dL) | Treatment | 176.77 ± 4.96 a | 150.13 ± 2.78 b | 152.53 ± 4.83 b | 0.001 | 0.001 | 0.03 |
| Post-treatment | 188.10 ± 4.06 a | 162.13 ± 5.30 b | 170.78 ± 6.20 b | 0.001 | 0.40 | 0.67 | |
| Triglycerides (mg/dL) | Treatment | 42.40 ± 1.02 | 39.87 ± 0.77 | 41.94 ± 0.77 | 0.08 | 0.04 | 0.37 |
| Post-treatment | 42.70 ± 1.28 | 44.00 ± 1.26 | 42.29 ± 1.14 | 0.59 | 0.52 | 0.28 | |
| Urea-N (mg/dL) | Treatment | 34.97 ± 0.66 | 33.52 ± 0.66 | 33.88 ± 0.68 | 0.21 | 0.001 | 0.48 |
| Post-treatment | 33.20 ± 0.95 | 33.62 ± 0.92 | 32.65 ± 0.83 | 0.75 | 0.93 | 0.22 | |
| Creatinine (mg/dL) | Treatment | 2.78 ± 0.09a | 2.22 ± 0.20 b | 2.36 ± 0.19 b | 0.001 | 0.001 | 0.001 |
| Post-treatment | 2.91 ± 0.11 | 3.10 ± 0.14 | 3.14 ± 0.09 | 0.25 | 0.04 | 0.06 | |
| Glucose (mg/dL) | Treatment | 85.18 ± 1.04 | 84.20 ± 0.94 | 83.73 ± 0.81 | 0.54 | 0.40 | 0.90 |
| Post-treatment | 83.66 ± 1.45 | 83.14 ± 1.50 | 83.78 ± 1.34 | 0.94 | 0.65 | 0.22 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Suwaiegh, S.B.; Morshedy, S.A.; Mansour, A.T.; Ahmed, M.H.; Zahran, S.M.; Alnemr, T.M.; Sallam, S.M.A. Effect of an Essential Oil Blend on Dairy Cow Performance during Treatment and Post-Treatment Periods. Sustainability 2020, 12, 9123. https://0-doi-org.brum.beds.ac.uk/10.3390/su12219123
Al-Suwaiegh SB, Morshedy SA, Mansour AT, Ahmed MH, Zahran SM, Alnemr TM, Sallam SMA. Effect of an Essential Oil Blend on Dairy Cow Performance during Treatment and Post-Treatment Periods. Sustainability. 2020; 12(21):9123. https://0-doi-org.brum.beds.ac.uk/10.3390/su12219123
Chicago/Turabian StyleAl-Suwaiegh, Shaker B., Sabrin A. Morshedy, Abdallah T. Mansour, Mohamed H. Ahmed, Soliman M. Zahran, Tareq M. Alnemr, and Sobhy M.A. Sallam. 2020. "Effect of an Essential Oil Blend on Dairy Cow Performance during Treatment and Post-Treatment Periods" Sustainability 12, no. 21: 9123. https://0-doi-org.brum.beds.ac.uk/10.3390/su12219123