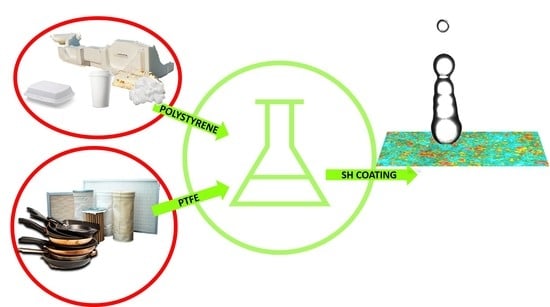
Superhydrophobicity and Durability in Recyclable Polymers Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Preparation and Characterization
2.2. Thermal Treatment
2.3. Wearing Test and Durability
2.4. Resistance to Acid and Base
2.5. Raining Test
3. Results
3.1. Surface Characterization and Wettability
3.2. Resistance to Acid and Base
3.3. Wearing Test
3.4. Thermal Treatment
3.5. Raining Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barthlott, W.; Ehler, N. Raster-Elektronenmikroskopie der Epidermis–Oberflächen von Spermatophyten. Akad. Wiss. Lit. 1977, 19, 367–465. [Google Scholar]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Wagner, T.; Neinhuis, C.; Barthlott, W. Wettability and Contaminability of Insect Wings as a Function of Their Surface Sculptures. Acta Zool. 1996, 77, 213–225. [Google Scholar] [CrossRef]
- Zhai, L.; Berg, M.C.; Cebeci, F.Ç.; Kim, Y.; Milwid, J.M.; Rubner, M.F.; Cohen, R.E. Patterned superhydrophobic surfaces: Toward a synthetic mimic of the namib desert beetle. Nano Lett. 2006, 6, 1213–1217. [Google Scholar] [CrossRef]
- Watson, G.S.; Gellender, M.; Watson, J.A. Self-propulsion of dew drops on lotus leaves: A potential mechanism for self-cleaning. Biofouling 2014, 30, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Bixler, G.D.; Bhushan, B. Fluid drag reduction and efficient self-cleaning with rice leaf and butterfly wing bioinspired surfaces. Nanoscale 2013, 5, 7685–7710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Zhang, H.; Wang, Z.; Liu, Y. Superhydrophobic aluminum alloy surface: Fabrication, structure, and corrosion resistance. Colloids Surf. Physicochem. Eng. Asp. 2014, 441, 319–325. [Google Scholar] [CrossRef]
- Jin, C.; Li, J.; Han, S.; Wang, J.; Sun, Q. A durable, superhydrophobic, superoleophobic and corrosion-resistant coating with rose-like ZnO nanoflowers on a bamboo surface. Appl. Surf. Sci. 2014, 320, 322–327. [Google Scholar] [CrossRef]
- Arnott, J.; Wu, A.H.F.; Vucko, M.J.; Lamb, R.N. Marine antifouling from thin air. Biofouling 2014, 30, 1045–1054. [Google Scholar] [CrossRef]
- Detty, M.R.; Drake, D.R.; Tang, Y.; Bright, F. Hybrid anti-fouling coating compositions and methods for preventing the fouling of surfaces subjected to a marine environment. U.S. Patent 7244295, 17 July 2007. [Google Scholar]
- Dong, H.; Cheng, M.; Zhang, Y.; Wei, H.; Shi, F. Extraordinary drag-reducing effect of a superhydrophobic coating on a macroscopic model ship at high speed. J. Mater. Chem. 2013, 1, 5886–5891. [Google Scholar] [CrossRef]
- Brassard, J.-D.; Sarkar, D.K.; Perron, J. Studies of drag on the nanocomposite superhydrophobic surfaces. Appl. Surf. Sci. 2015, 324, 525–531. [Google Scholar] [CrossRef]
- Pu, X.; Li, G.; Huang, H. Preparation, anti-biofouling and drag-reduction properties of a biomimetic shark skin surface. Biol. Open 2016, 5, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Privett, B.J.; Youn, J.; Hong, S.a.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinonen, S.; Huttunen-Saarivirta, E.; Nikkanen, J.-P.; Raulio, M.; Priha, O.; Laakso, J.; Storgårds, E.; Levänen, E. Antibacterial properties and chemical stability of superhydrophobic silver-containing surface produced by sol-gel route. Colloids Surf. Physicochem. Eng. Asp. 2014, 453, 149–161. [Google Scholar] [CrossRef]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-Icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Song, Y.; Jiang, L.; Wang, J. Bio-inspired strategies for anti-icing. ACS Nano 2014, 8, 3152–3169. [Google Scholar] [CrossRef]
- Ruan, M.; Zhan, Y.; Wu, Y.; Wang, X.; Li, W.; Chen, Y.; Wei, M.; Wang, X.; Deng, X. Preparation of PTFE/PDMS superhydrophobic coating and its anti-icing performance. RSC Adv. 2017, 7, 41339–41344. [Google Scholar] [CrossRef] [Green Version]
- Ying, J.; Eid, K.F.; Sommers, A.D. Designing Energy-Efficient Heat Exchangers—Creating Micro-Channels On The Aluminum Fin Surface. Int. Refrig. Air Cond. Conf. 2010, 2836, 1–8. [Google Scholar]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Ishizaki, T.; Hieda, J.; Saito, N.; Saito, N.; Takai, O. Corrosion resistance and chemical stability of super-hydrophobic film deposited on magnesium alloy AZ31 by microwave plasma-enhanced chemical vapor deposition. Electrochim. Acta 2010, 55, 7094–7101. [Google Scholar] [CrossRef]
- Vilaró, I.; Yagüe, J.L.; Borros, S. Superhydrophobic copper surfaces with anti-corrosion properties fabricated by solventless CVD methods. ACS Appl. Mater. Interfaces 2016, 9, 1057–1065. [Google Scholar] [CrossRef]
- Li, B.J.; Li, H.; Huang, L.J.; Ren, N.F.; Kong, X. Femtosecond pulsed laser textured titanium surfaces with stable superhydrophilicity and superhydrophobicity. Appl. Surf. Sci. 2016, 389, 585–593. [Google Scholar] [CrossRef]
- Wu, L.K.; Hu, J.M.; Zhang, J.Q. One step sol–gel electrochemistry for the fabrication ofsuperhydrophobic surfaces. J. Mater. Chem. 2013, 1, 14471–14475. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, F.; Ye, L.; Xiong, P.; Yan, L.; Yang, W.; Jiang, B. A one-pot sol–gel process to prepare a superhydrophobic and environment-resistant thin film from ORMOSIL nanoparticles. RSC Adv. 2014, 4, 9838. [Google Scholar] [CrossRef]
- Wang, J.; Ren, K.; Chang, H.; Zhang, S.; Jin, L.; Ji, J. Facile fabrication of robust superhydrophobic multilayered film based on bioinspired poly(dopamine)-modified carbon nanotubes. Phys. Chem. Chem. Phys. 2014, 16, 2936–2943. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Yao, K. Facile Fabrication of Superhydrophobic Surface with Excellent Mechanical Abrasion and Corrosion Resistance on Copper Substrate by a Novel Method. ACS Appl. Mater. Interfaces 2014, 6, 8762–8770. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Li, Y.; Xu, C. Highly transparent and durable superhydrophobic hybrid nanoporous coatings fabricated from polysiloxane. ACS Appl. Mater. Interfaces 2014, 6, 10014–10021. [Google Scholar] [CrossRef] [PubMed]
- Pi, P.; Mu, W.; Fei, G.; Deng, Y. Superhydrophobic film fabricated by controlled microphase separation of PEO–PLA mixture and its transparence property. Appl. Surf. Sci. 2013, 273, 184–191. [Google Scholar] [CrossRef]
- Davaasuren, G.; Ngo, C.V.; Oh, H.S.; Chun, D.M. Geometric study of transparent superhydrophobic surfaces of molded and grid patterned polydimethylsiloxane. Appl. Surf. Sci. 2014, 314, 530–536. [Google Scholar] [CrossRef]
- Wang, M.F.; Raghunathan, N.; Ziaie, B. A nonlithographic top-down electrochemical approach for creating hierarchical (micro-nano) superhydrophobic silicon surfaces. Langmuir 2007, 23, 2300–2303. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tuominen, M.T.; Rothstein, J.P. Hierarchical superhydrophobic surfaces fabricated by dual-scale electron-beam-lithography with well-ordered secondary nanostructures. Adv. Funct. Mater. 2011, 21, 3715–3722. [Google Scholar] [CrossRef]
- Wong, H.S.; Barakat, R.; Alhilali, A.; Saleh, M.; Cheeseman, C.R. Hydrophobic concrete using waste paper sludge ash. Cem. Concr. Res. 2015, 70, 9–20. [Google Scholar] [CrossRef]
- Shen, L.; Qiu, W.; Liu, B.; Guo, Q. Stable superhydrophobic surface based on silicone combustion product. RSC Adv. 2014, 4, 56259–56262. [Google Scholar] [CrossRef]
- Guo, L.P.; Sun, W.; Yu, T.; Feng, M.Z. Preparation and characteristics of a recycled cement-based superhydrophobic coating with dirt pickup resistance. Sci. China Technol. Sci. 2015, 58, 1096–1104. [Google Scholar] [CrossRef]
- Wen, G.; Huang, J.X.; Guo, Z.G. Energy-effective superhydrophobic nanocoating based on recycled eggshell. Colloids Surf. Physicochem. Eng. Asp. 2019, 568, 20–28. [Google Scholar] [CrossRef]
- Ray, S.S.; Gandhi, M.; Chen, S.S.; Chang, H.M.; Dan, C.T.N.; Le, H.Q. Anti-wetting behaviour of a superhydrophobic octadecyltrimethoxysilane blended PVDF/recycled carbon black composite membrane for enhanced desalination. Environ. Sci. Water Res. Technol. 2018, 4, 1612–1623. [Google Scholar] [CrossRef]
- Lin, Y.T.; Chou, J.H. Fabricating curved super-hydrophobic surfaces greenly using recycled polypropylene. Int. J. Plast. Technol. 2016, 20, 1–10. [Google Scholar] [CrossRef]
- Accudynetest. Critical Surface Tension and Contact Angle with Water for Various Polymers. 2009. Available online: https://www.accudynetest.com/polytable_03.html?sortby=contact_angle (accessed on 13 September 2019).
- Salapare, H.S.; Guittard, F.; Noblin, X.; de Givenchy, E.T.; Celestini, F.; Ramos, H.J. Stability of the hydrophilic and superhydrophobic properties of oxygen plasma-treated poly(tetrafluoroethylene) surfaces. J. Colloid Interface Sci. 2013, 396, 287–292. [Google Scholar] [CrossRef]
- Zhu, Y.; He, Y.; Zhang, J.F.; Li, Z.H.; Zhou, W.; Yang, D.Q.; Sacher, E. Preparation of large-scale, durable, superhydrophobic PTFE films using rough glass templates. Surf. Interface Anal. 2017, 49, 1422–1430. [Google Scholar] [CrossRef]
- Jiang, C.; Hou, W.; Wang, Q.; Wang, T. Facile fabrication of superhydrophobic polytetrafluoroethylene surface by cold pressing and sintering. Appl. Surf. Sci. 2011, 257, 4821–4825. [Google Scholar] [CrossRef]
- Nilsson, M.A.; Daniello, R.J.; Rothstein, J.P. A novel and inexpensive technique for creating superhydrophobic surfaces using Teflon and sandpaper. J. Phys. Appl. Phys. 2010, 43, 045301. [Google Scholar] [CrossRef]
- Zhan, Y.L.; Ruan, M.; Li, W.; Li, H.; Hu, L.Y.; Ma, F.M.; Yu, Z.L.; Feng, W. Fabrication of anisotropic PTFE superhydrophobic surfaces using laser microprocessing and their self-cleaning and anti-icing behavior. Colloids Surf. Physicochem. Eng. Asp. 2017, 535, 8–15. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.; Yu, C.; Li, K.; Tian, Y.; Jiang, L. In Situ Separation of Chemical Reaction Systems Based on a Special Wettable PTFE Membrane. Adv. Funct. Mater. 2018, 28, 1703970. [Google Scholar] [CrossRef]
- Fang, Y.; Yong, J.; Chen, F.; Huo, J.; Yang, Q.; Bian, H.; Du, G.; Hou, X. Durability of the tunable adhesive superhydrophobic PTFE surfaces for harsh environment applications. Appl. Phys. 2016, 122, 827. [Google Scholar] [CrossRef]
- Kecskeméti, G.; Hopp, B.; Smausz, T.; Tóth, Z.; Szabó, G. Production of porous PTFE-Ag composite thin films by pulsed laser deposition. Appl. Surf. Sci. 2012, 258, 7982–7988. [Google Scholar] [CrossRef]
- Bodas, D.S.; Mandale, A.B.; Gangal, S.A. Deposition of PTFE thin films by RF plasma sputtering on ⟨1 0 0⟩ silicon substrates. Appl. Surf. Sci. 2005, 245, 202–207. [Google Scholar] [CrossRef]
- Tripathi, S.; De, R.; Haque, S.M.; Rao, K.D.; Misal, J.S.; Prathap, C.; Das, S.C.; Patidar, M.M.; Ganesan, V.; Sahoo, N.K. Annealing dependent evolution of columnar nanostructures in RF magnetron sputtered PTFE films for hydrophobic applications. Mater. Res. Express. 2018, 5, 015312. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhang, T.; Zhang, X.; Xin, Z.; Prakashan, K. Fabrication of a thin-layer PTFE coating exhibiting superhydrophobicity by supercritical CO2. Prog. Org. Coat. 2017, 111, 322–326. [Google Scholar] [CrossRef]
- Qing, W.; Shi, X.; Deng, Y.; Zhang, W.; Wang, J.; Tang, C.Y. Robust superhydrophobic-superoleophilic polytetrafluoroethylene nanofibrous membrane for oil/water separation. J. Membr. Sci. 2017, 540, 354–361. [Google Scholar] [CrossRef]
- Zhuang, A.; Liao, R.; Dixon, S.C.; Lu, Y.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Transparent superhydrophobic PTFE films via one-step aerosol assisted chemical vapor deposition. RSC Adv. 2017, 7, 29275–29283. [Google Scholar] [CrossRef] [Green Version]
- Lei, S.; Shi, Z.; Ou, J.; Li, W.; Qiao, G.; Yu, X. A facile process for preparing superhydrophobic PBZ-PTFE coating with excellent stable properties. Appl. Phys. 2016, 122, 1011. [Google Scholar] [CrossRef]
- Weng, R.; Zhang, H.; Liu, X. Spray-coating process in preparing PTFE-PPS composite super-hydrophobic coating. AIP Adv. 2014, 4, 031327. [Google Scholar] [CrossRef] [Green Version]
- Pawar, P.G.; Xing, R.; Kambale, R.C.; Kumar, A.M.; Liu, S.; Latthe, S.S. Polystyrene assisted superhydrophobic silica coatings with surface protection and self-cleaning approach. Prog. Org. Coat. 2017, 105, 235–244. [Google Scholar] [CrossRef]
- Liggieri, L.; Passerone, A. An automatic technique for measuring the surface tension of liquid metals. High Temp. Technol. 1989, 7, 82–86. [Google Scholar] [CrossRef]
- Kumudine, C.; Premachandra, J. Polymer Data Handbook; American Chemical Society: Washington, DC, USA, 1999. [Google Scholar]
- Michigan Department of Environmental Quality. Michigan’s Water Chemistry Monitoring Program; EGLE: Lansing, MI, USA, 2013.
- Met Office. The Beaufort Scale; National Meteorological Library and Archive: London, UK, 2010; pp. 1–22.
- Li, K.; Zeng, X.; Li, H.; Lai, X. Fabrication and characterization of stable superhydrophobic fluorinated-polyacrylate/silica hybrid coating. Appl. Surf. Sci. 2014, 298, 214–220. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Zhu, Y.; Li, Z.; Sheng, T.; Hu, Y.M.; Yang, D.Q. A study of the mechanical and chemical durability of Ultra-Ever Dry Superhydrophobic coating on low carbon steel surface. Colloids Surf. Physicochem. Eng. Asp. 2016, 497, 16–27. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, Z.; Zhao, L.; Dong, B.; Wang, S. Fabrication of transparent, durable and self-cleaning superhydrophobic coatings for solar cells. New J. Chem. 2020, 44, 14481–14489. [Google Scholar] [CrossRef]
- Huang, W.-H.; Lin, C.-S. Robust superhydrophobic transparent coatings fabricated by a low-temperature sol–gel process. Appl. Surf. Sci. 2014, 305, 702–709. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, Z.; Ben, K.; Guan, Z. A self-modification approach toward transparent superhydrophobic glass for rainproofing and superhydrophobic fiberglass mesh for oil–water separation. Appl. Surf. Sci. 2016, 360, 789–797. [Google Scholar] [CrossRef]
- Xu, Q.F.; Wang, J.N.; Smith, I.H.; Sanderson, K.D. Superhydrophobic and transparent coatings based on removable polymeric spheres. J. Mater. Chem. 2009, 19, 655–660. [Google Scholar] [CrossRef]
- Labware Chemical Resistance Table; Thermo Fisher Scientific Inc: Waltham, MA, USA, 2015; pp. 1–15.
- Zhu, W.; Liu, H.; Yan, W.; Chen, T. The fabrication of superhydrophobic PTFE/UHMWPE composite surface by hot-pressing and texturing process. Colloid Polym. Sci. 2017, 295, 759–766. [Google Scholar] [CrossRef]
- Burkarter, E.; Saul, C.K.; Thomazi, F.; Cruz, N.C.; Roman, L.S.; Schreiner, W.H. Superhydrophobic electrosprayed PTFE. Surf. Coat. Technol. 2007, 202, 194–198. [Google Scholar] [CrossRef]
- Young, T.J.; Jackson, J.; Roy, S.; Ceylan, H.; Sundararajan, S. Tribological behavior and wettability of spray-coated superhydrophobic coatings on aluminum. Wear 2017, 376, 1713–1719. [Google Scholar] [CrossRef]
- Baba, E.M.; Cansoy, C.E.; Zayim, E.O. Investigation of wettability and optical properties of superhydrophobic polystyrene-SiO2 composite surfaces. Prog. Org. Coat. 2016, 99, 378–385. [Google Scholar] [CrossRef]







| Time (h) | T (°C) |
|---|---|
| 3 | 200 |
| 10 | 200 |
| 30 | 200 |
| 1 | 250 |
| 1 | 280 |
| 30 | 280 |
| 60 | 280 |
| 1 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cirisano, F.; Ferrari, M. Superhydrophobicity and Durability in Recyclable Polymers Coating. Sustainability 2021, 13, 8244. https://0-doi-org.brum.beds.ac.uk/10.3390/su13158244
Cirisano F, Ferrari M. Superhydrophobicity and Durability in Recyclable Polymers Coating. Sustainability. 2021; 13(15):8244. https://0-doi-org.brum.beds.ac.uk/10.3390/su13158244
Chicago/Turabian StyleCirisano, Francesca, and Michele Ferrari. 2021. "Superhydrophobicity and Durability in Recyclable Polymers Coating" Sustainability 13, no. 15: 8244. https://0-doi-org.brum.beds.ac.uk/10.3390/su13158244