Growth Promotion of Guava “Pear” (Psidium guajava cv.) by Sinorhizobium mexicanum in Southern Mexican Agricultural Fields
Abstract
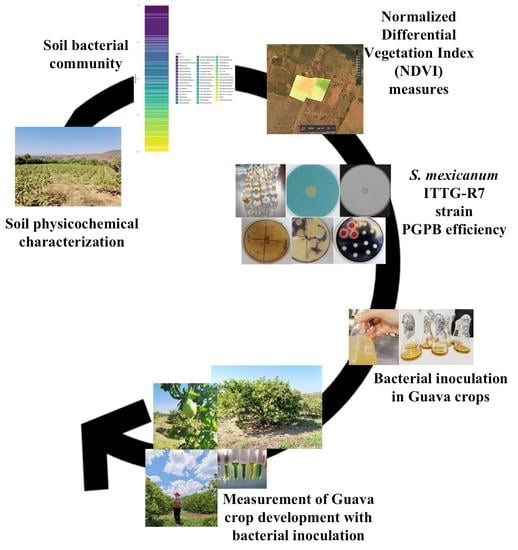
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bacterial Strain
2.3. Soil Physicochemical Analyses
2.4. Assessment of the Bacterial Community in Guava Agricultural Soil
2.5. Sinorhizobium mexicanum ITTG-R7T as PGPB
2.6. Experimental Design for Field Inoculation
2.7. Bacterial Inoculation in Guava Crops
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Agricultural Field
3.2. Physicochemical Soil Characteristics
3.3. Bacterial Community Structure
3.4. PGPB Efficiency by Sinorhizobium ITTG-R7T Strain
3.5. Effect of Bacterial Inoculation in Guava Development
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borlaug, N.E. Feeding a world of 10 billion people: The miracle ahead. In Vitro Cellular & Developmental Biology. Plant 2002, 38, 221–228. [Google Scholar] [CrossRef]
- Mozumder, P.; Berrens, R.P. Inorganic fertilizer use and biodiversity risk: An empirical investigation. Ecol. Econ. 2007, 62, 538–543. [Google Scholar] [CrossRef]
- Xuejun, L.; Fusuo, Z. Nitrogen fertilizer induced greenhouse gas emissions in China. Curr. Opin. Environ. Sustain. 2011, 3, 407–413. [Google Scholar] [CrossRef]
- Kukla, J.; Whitfeld, T.; Cajthaml, T.; Baldrian, P.; Veselá-Šimáčková, H.; Novotný, V.; Frouz, J. The effect of traditional slash-and-burn agriculture on soil organic matter, nutrient content, and microbiota in tropical ecosystems of Papua New Guinea. Land Degrad. Dev. 2019, 30, 166–177. [Google Scholar] [CrossRef]
- Singh, V.; Shukla, S.; Singh, A. The principal factors responsible for biodiversity loss. Open J. Plant Sci. 2021, 6, 11–14. [Google Scholar]
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afr. J. Biotechnol. 2011, 10, 19840–19849. [Google Scholar] [CrossRef]
- Rafi, M.M.; Krishnaveni, M.S.; Charyulu, P.B.B.N. Phosphate-solubilizing microorganisms and their emerging role in sustainable agriculture. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Dordrecht, The Netherlands, 2019; p. 223. [Google Scholar] [CrossRef]
- Mącik, M.; Gryta, A.; Frąc, M. Biofertilizers in agriculture: An overview on concepts, strategies and effects on soil microorganisms. Adv. Agron. 2020, 162, 31–87. [Google Scholar]
- Subrahmanyam, G.; Kumar, A.; Luikham, R.; Kumar, J.S.; Yadav, A.N. Global Scenario of Soil Microbiome Research: Current Trends and Future Prospects. In Soil Microbiomes for Sustainable Agriculture; Springer: Cham, Switzerland, 2021; pp. 573–603. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant growth-promoting microorganisms for environmental sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef]
- Sharma, S.; Rana, V.S.; Kumari, M.; Mishra, P. Biofertilizers: Boon for fruit production. J. Pharm. Phytochem. 2018, 7, 3244–3247. [Google Scholar]
- Lopes, M.J.D.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful plant growth-promoting microbes: Inoculation methods and abiotic factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Rincón-Molina, C.I.; Martínez-Romero, E.; Ruiz-Valdiviezo, V.M.; Velázquez, E.; Ruiz-Lau, N.; Rogel-Hernández, M.A.; Villalobos-Maldonado, J.J.; Rincón-Rosales, R. Plant growth-promoting potential of bacteria associated to pioneer plants from an active volcanic site of Chiapas (Mexico). Appl. Soil Ecol. 2020, 146, 103390. [Google Scholar] [CrossRef]
- De Bruijn, F.J. Biological nitrogen fixation. In Principles of Plant-Microbe Interactions; Springer: Cham, Switzerland, 2015; pp. 215–224. [Google Scholar]
- Soumare, A.; Diedhiou, A.G.; Thuita, M.; Hafidi, M.; Ouhdouch, Y.; Gopalakrishnan, S.; Kouisni, L. Exploiting biological nitrogen fixation: A route towards a sustainable agriculture. Plants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Moulin, L.; Bontemps, C.; Vandamme, P.; Béna, G.; Boivin-Masson, C. Legume Symbiotic Nitrogen Fixation by β-Proteobacteria Is Widespread in Nature. J. Bacteriol. 2003, 185, 7266–7272. [Google Scholar] [CrossRef]
- Sawada, H.; Kuykendall, L.D.; Young, J.M. Changing concepts in the systematics of bacterial nitrogen-fixing legume symbionts. The Journal of general and applied microbiology. J. Gen. Appl. Microbiol. 2002, 49, 155–179. [Google Scholar] [CrossRef] [PubMed]
- Shridhar, B.S. Nitrogen fixing microorganisms. Int. J. Microbiol. Res. 2012, 3, 46–52. [Google Scholar]
- Lloret, L.; Ormeno-Orrillo, E.; Rincón-Rosales, R.; Martínez-Romero, J.; Rogel-Hernández, M.A.; Martínez-Romero, E. Ensifer mexicanus sp. nov. a new species nodulating Acacia angustissima (Mill.) Kuntze in Mexico. Syst. Appl. Microbiol. 2007, 30, 280–290. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Lloret, L.; Ponce, E.; Martínez-Romero, E. Rhizobia with different symbiotic efficiencies nodulate Acaciella angustissima in Mexico, including Sinorhizobium chiapanecum sp. nov. which has common symbiotic genes with Sinorhizobium mexicanum. FEMS Microbiol. Ecol. 2009, 67, 103–117. [Google Scholar] [CrossRef]
- Rincón-Rosales, R.; Ruiz-Valdiviezo, V.M.; Montes-Molina, J.A.; Gutierrez-Miceli, F.A.; Dendooven, L. Aluminium tolerance in the tropical leguminous N2-fixing shrub Acaciella angustissima (Mill.) Britton & Rose inoculated with Sinorhizobium mexicanum. Gayana Bot. 2011, 68, 188–195. [Google Scholar]
- Rincón-Rosales, R.; Rogel, M.A.; Guerrero, G.; Rincón-Molina, C.I.; Lopez-Lopez, A.; Manzano-Gómez, L.A.; Ruiz-Valdiviezo, V.M.; Martinez-Romero, E. Genomic Data of Acaciella Nodule Ensifer mexicanus ITTG R7T. Microbiol. Resour. Announc. 2021, 10, e01251-20. [Google Scholar] [CrossRef]
- Devi, H.L.; Mitra, S.K.; Poi, S.C. Effect of different organic and biofertilizer sources on guava (Psidium guajava L.) ‘Sardar’. In Proceedings of the III International Symposium on Guava and other Myrtaceae, Petrolina, Brazil, 23–25 April 2012; Volume 959, pp. 201–208. [Google Scholar]
- Shukla, S.K.; Adak, T.; Singha, A.; Kumar, K.; Singh, V.K.; Singh, A. Response of guava trees (Psidium guajava) to soil applications of mineral and organic fertilisers and biofertilisers under conditions of low fertile soil. J. Agric. Res. 2014, 22, 105–114. [Google Scholar] [CrossRef]
- Manzano-Gómez, L.A.; Guzmán-Albores, J.M.; Rincón-Rosales, R.; Winkler, R.; Rincón-Molina, C.I.; Castañón-González, J.H.; Ruiz Lau, N.; Gutiérrez-Miceli, F.A.; Rincón-Molina, F.A.; Ruíz-Valdiviezo, V.M. Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types. Agronomy 2021, 11, 2061. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Kolde, R. pheatmap: Pretty Heatmaps. R Package Version 1.0.8. 2015. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 10 December 2021).
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhang, S.; Fu, Y.; Fan, X.; Patel, J.; Zhang, M. Characterization of phosphate-solubilizing bacteria isolated from calcareous soils. Appl. Soil Ecol. 2015, 96, 217–224. [Google Scholar] [CrossRef]
- Brick, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid in situ assay for indole acetic acid production by bacteria immobilized on nitrocellulose membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Rizo, J.; Rogel, M.A.; Guillén, D.; Wacher, C.; Martínez-Romero, E.; Encarnación, S.; Rodríguez-Sanoja, R. Nitrogen fixation in pozol, a traditional fermented beverage. Appl. Environ. Microbiol. 2020, 86, e00588-20. [Google Scholar] [CrossRef]
- Glick, B.R. The enhancement of plant growth by free living bacteria. Can. J. Microbiol. 1995, 41, 109–114. [Google Scholar] [CrossRef]
- Amaresan, N.; Kumar, K.; Sureshbabu, K.; Madhuri, K. Plant growth promoting potential of bacteria isolated from active volcano sites of Barren Island, India. Lett. Appl. Microbiol. 2013, 58, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, N.; Gupta, A.; Khare, S.K. Production of protease and lipase by solvent tolerant Pseudomonas aeruginosa PseA in solid-state fermentation using Jatropha curcas seed cake as substrate. Bioresour. Technol. Rep. 2008, 99, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, R.; Naili, B.; Bejar, S. Application of a statistical design to the optimization of parameters and culture medium for a-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour. Technol. 2008, 99, 5602–5609. [Google Scholar] [CrossRef] [PubMed]
- Dastager, S.G.; Pandey, A.; Lee, J.C.; Li, W.J.; Kim, C.J. Polyphasic taxonomy of novel actinobacteria showing macromolecule degradation potentials in Bigeum Island, Korea. Curr. Microbiol. 2009, 59, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kasana, R.C.; Salwan, R.; Dhar, H.; Dutt, S.; Gulati, A. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr. Microbiol. 2008, 57, 503–507. [Google Scholar] [CrossRef]
- Rani, M.; Kaur, G.; Kaur, K.; Arora, N.K. Effect of organic manures and biofertilizers on growth, fruit quality and leaf nutrient status of guava. Agric. Res. J. 2021, 58, 835–839. [Google Scholar] [CrossRef]
- Ruiz-Valdiviezo, V.M.; Ayora-Talavera, T.R.; Gutiérrez-Miceli, F.A.; Dendooven, L.; Rincón-Rosales, R. Effects of inorganic fertilizers and rhizobial inoculation on growth, nodulation and tannin content Acaciella angustissima (Mill.) Britton & Rose. Gayana Bot. 2009, 66, 206–217. [Google Scholar]
- Chang, W.S.; Lee, H.I.; Hungria, M. Soybean Production in the Americas. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- García-Fraile, P.; Carro, L.; Robledo, M.; Ramírez-Bahena, M.H.; Flores-Félix, J.D.; Fernández, M.T.; Velázquez, E. Rhizobium promotes non-legumes growth and quality in several production steps: Towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 2012, 7, e38122. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Ormeño-Orrillo, E.; López-López, A.; Rogel, M.A.; Reyes-Hernández, B.J.; Martínez-Romero, J.C.; Martínez-Romero, E. Nitrogen fixation in cereals. Front. Microbiol. 2018, 9, 1794. [Google Scholar] [CrossRef]
- Gómez-Godínez, L.J.; Fernández-Valverde, S.L.; Martínez Romero, J.C.; Martínez-Romero, E. Metatranscriptomics and nitrogen fixation from the rhizoplane of maize plantlets inoculated with a group of PGPRs. Syst. Appl. Microbiol. 2019, 42, 517–525. [Google Scholar] [CrossRef]
- Leite, R.D.C.; dos Santos, J.G.; Silva, E.L.; Alves, C.R.; Hungria, M.; Leite, R.D.C.; dos Santos, A.C. Productivity increase, reduction of nitrogen fertiliser use and drought-stress mitigation by inoculation of Marandu grass (Urochloa brizantha) with Azospirillum brasilense. Crop Pasture Sci. 2018, 70, 61–67. [Google Scholar] [CrossRef]
- Kundu, S.; Datta, P.; Mishra, J.; Rashmi, K.; Ghosh, B. Influence of biofertilizer and inorganic fertilizer in pruned mango orchard cv. Amrapali. J. Crop Weed Sci. 2011, 7, 100–103. [Google Scholar]
- Dwivedi, D.H.; Lata, R.; Ram, R.B.; Babu, M. Effect of biofertilizer and organic manures on yield and quality of Red Fleshed Guava. Acta Hort. 2012, 933, 239–244. [Google Scholar] [CrossRef]
- Khalil, N.H.; Agah, R.J. Effect of Chemical, Organic and Bio Fertilization on Growth and Yield of Strawberry Plant. Int. J. Adv. Chem. Eng. Biol. Sci. 2017, 4, 5. [Google Scholar]
- Durigon, V.L.; Carvalho, D.F.; Antunes, M.A.H.; Oliveira, P.T.S.; Fernandes, M.M. NDVI time series for monitoring RUSLE cover management factor in a tropical watershed. Int. J. Remote Sens. 2014, 35, 441–453. [Google Scholar] [CrossRef]
- Rousta, I.; Olafsson, H.; Moniruzzaman, M.; Zhang, H.; Liou, Y.A.; Mushore, T.D.; Gupta, A. Impacts of drought on vegetation assessed by vegetation indices and meteorological factors in Afghanistan. Remote Sens. 2020, 12, 2433. [Google Scholar] [CrossRef]
- Inoue, Y. Satellite-and drone-based remote sensing of crops and soils for smart farming–a review. Soil Sci. Plant Nutr. 2020, 66, 798–810. [Google Scholar] [CrossRef]
- Andrade, J.F.; Ermacora, M.; Satorre, E.H. Assessing benefits of land use intensification on extensive grain cropping systems of the Pampas. Eur. J. Agron. 2022, 135, 126484. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, J.; Zhu, L. Evaluation of the application potential of bentonites in phenanthrene bioremediation by characterizing the biofilm community. Bioresour. Technol. 2013, 134, 17–23. [Google Scholar] [CrossRef]
- Oh, S.; Choi, D. Microbial community enhances biodegradation of bisphenol a through selection of Sphingomonadaceae. Microb. Ecol. 2019, 77, 631–639. [Google Scholar] [CrossRef]
- Ding, P.; Chu, L.; Wang, J. Advanced treatment of petrochemical wastewater by combined ozonation and biological aerated filter. Environ. Sci. Pollut. Res. 2018, 25, 9673–9682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, B.; Gao, Y.; Wang, Y.; Lu, J.; Chen, J.; Deng, Q. The role of available phosphorous in vanadate decontamination by soil indigenous microbial consortia. Environ. Pollut. 2021, 289, 117839. [Google Scholar] [CrossRef] [PubMed]



| Strain | P Solubilization Index | IAA | ARA ¥ | ACC Deaminase | Extracellular Enzymes | ||||
|---|---|---|---|---|---|---|---|---|---|
| Siderophore | Protease | Amylase | Lipase | Cellulase | |||||
| S. mexicanum ITTG-R7T | 2.98 ± (0.10) ≠ | + | 3.21 ± (0.16) | + | + | + | + | + | + |
| A. brasilense CD | 2.42 ± (0.52) | + | 2.92 ± (0.23) | + | + | − | + | − | + |
| Treatment | Total Plant Height (cm) | Foliar Cover (cm) | Basal Diameter (cm) | Number of Flowers | Number of Fruits | Total Chlorophyll (mg mL−1) |
|---|---|---|---|---|---|---|
| T1: [S. mexicanum ITTG-R7T] | 368.83 A * | 553.16 A | 97.83 AB | 36.0 A | 63.33 A | 2.81 A |
| T2: [S. mexicanum ITTG-R7T + Triple 17 + Diammonium phosphate + Nitrabor] | 327.5 B | 516.83 AB | 107.16 A | 29.16 B | 52.66 AB | 3.70 B |
| T3: [A. brasilense CD] | 312.67 B | 462.33 B | 64.0 C | 21.66 C | 34.83 BC | 2.65 B |
| T4: [Triple 17 + Diammonium phosphate + Nitrabor] | 336.33 B | 475.5 B | 86.16 B | 23.5 BC | 50.33 AB | 2.70 B |
| T5: [Control (non-inoculated, non-fertilized)] | 267.5 C | 375.0 C | 57.0 C | 19.0 C | 24.83 C | 2.04 C |
| p-value | 0.0000 | 0.0000 | 0.0000 | 0.0036 | 0.0001 | 0.0000 |
| HSD £ (p <0.05) | 24.7275 | 58.4896 | 12.0903 | 6.7299 | 20.0775 | 0.3114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón-Molina, C.I.; Martínez-Romero, E.; Manzano-Gómez, L.A.; Rincón-Rosales, R. Growth Promotion of Guava “Pear” (Psidium guajava cv.) by Sinorhizobium mexicanum in Southern Mexican Agricultural Fields. Sustainability 2022, 14, 12391. https://0-doi-org.brum.beds.ac.uk/10.3390/su141912391
Rincón-Molina CI, Martínez-Romero E, Manzano-Gómez LA, Rincón-Rosales R. Growth Promotion of Guava “Pear” (Psidium guajava cv.) by Sinorhizobium mexicanum in Southern Mexican Agricultural Fields. Sustainability. 2022; 14(19):12391. https://0-doi-org.brum.beds.ac.uk/10.3390/su141912391
Chicago/Turabian StyleRincón-Molina, Clara Ivette, Esperanza Martínez-Romero, Luis Alberto Manzano-Gómez, and Reiner Rincón-Rosales. 2022. "Growth Promotion of Guava “Pear” (Psidium guajava cv.) by Sinorhizobium mexicanum in Southern Mexican Agricultural Fields" Sustainability 14, no. 19: 12391. https://0-doi-org.brum.beds.ac.uk/10.3390/su141912391