Human Cytotoxicity, Hemolytic Activity, Anti-Inflammatory Activity and Aqueous Solubility of Ibuprofen-Based Ionic Liquids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nuclear Magnetic Resonance (NMR)
2.3. Differential Scanning Calorimetry (DSC)
2.4. Solubility Studies
2.5. Cytotoxicity Assays
2.6. Hemolytic Activity
2.7. Protein Albumin Denaturation Assay
2.8. Cyclooxygenases (COX-1 and COX-2) Imhibition Assays
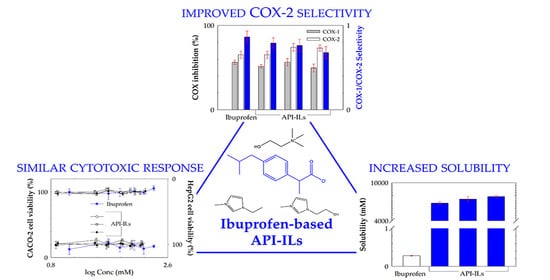
3. Results
3.1. Characterization of the Ibuprofen-Based Ionic Liquids
3.2. Equilibrium Solubility in Water and Simulated Biological Fluids
3.3. Cytotoxicity Profile in Human Cell Lines
3.4. Hemolytic Activity
3.5. Protein Albumin Denaturation Assay
3.6. Cyclooxygenases (COX-1 and COX-2) Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.; Tiwari, R.; Tiwari, G.; Yadav, P.; Shukla, P. Pharmaceutical polymorphism: The phenomenon affecting the performance of drug and an approach to enhance drug solubility, stability and bioavailability. World J. Pharm. Sci. 2016, 4, 411–419. [Google Scholar]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2020, 5, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.; Freire, M.G. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Seddon, K.R. Ionic Liquids—Progress on the Fundamental Issues. Aust. J. Chem. 2007, 60, 3–5. [Google Scholar] [CrossRef]
- Ferraz, R.; Branco, L.C.; Marrucho, I.M.; Araújo, J.M.M.; Rebelo, L.P.N.; da Ponte, M.N.; Prudêncio, C.; Noronha, J.P.; Petrovski, Z. Development of novel ionic liquids based on ampicillin. Med. Chem. Commun. 2012, 3, 494–497. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Stolte, S.; Araújo, J.M.M.; Rebelo, L.P.N.; Pereiro, A.B.; Markiewicz, M. Acute Aquatic Toxicity and Biodegradability of Fluorinated Ionic Liquids. ACS Sustain. Chem. Eng. 2019, 7, 3733–3741. [Google Scholar] [CrossRef]
- Vieira, N.S.M.; Bastos, J.C.; Rebelo, L.P.N.; Matias, A.; Araújo, J.M.M.; Pereiro, A.B. Human cytotoxicity and octanol/water partition coefficients of fluorinated ionic liquids. Chemosphere 2019, 216, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Vieira, N.S.; Castro, P.J.; Marques, D.F.; Araújo, J.M.M.; Pereiro, A.B. Tailor-Made Fluorinated Ionic Liquids for Protein Delivery. Nanomaterials 2020, 10, 1594. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Hachem, K.; Bokov, D.; Ansari, M.J.; Nakhjiri, A.T. Ionic liquids in pharmaceutical industry: A systematic review on applications and future perspectives. J. Mol. Liq. 2022, 349, 118145. [Google Scholar] [CrossRef]
- Balk, A.; Widmer, T.; Wiest, J.; Bruhn, H.; Rybak, J.C.; Matthes, P.; Müller-Buschbaum, K.; Sakalis, A.; Lühmann, T.; Berghausen, J.; et al. Ionic liquid versus prodrug strategy to address formulation challenges. Pharm. Research. 2015, 32, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.M.M.; Florindo, C.; Pereiro, A.B.; Vieira, N.S.M.; Matias, A.A.; Duarte, C.M.M.; Rebelo, P.N.; Marrucho, I.M. Cholinium-based ionic liquids with pharmaceutically active anions. RSC Adv. 2014, 4, 28126–28132. [Google Scholar] [CrossRef]
- Fernández-Stefanuto, V.; Esteiro, P.; Santiago, R.; Moreno, D.; Palomar, J.; Tojo, E. Design and synthesis of alverine-based ionic liquids to improve drug water solubility. New J. Chem. 2020, 44, 20428–20433. [Google Scholar] [CrossRef]
- Florindo, C.; Araújo, J.M.M.; Alves, F.; Matos, C.; Ferraz, R.; Prudêncio, C.; Marrucho, I.M. Evaluation of solubility and partition properties of ampicillin-based ionic liquids. Int. J. Pharm. 2013, 456, 553–559. [Google Scholar] [CrossRef]
- Zhao, H.; Holmes, S.S.; Baker, G.A.; Challa, S.; Bose, H.S.; Song, Z. Ionic derivatives of betulinic acid as novel HIV-1 protease inhibitors. J. Enzyme Inhib. Med. Chem. 2012, 27, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, M.; Onnis, V.; Zucca, P.; Rescigno, A.; Lachowicz, J.I.; Engelbrecht, L.D.V.; Nieddu, M.; Ennas, G.; Scano, A.; Mocci, F.; et al. Cholinium-Based Ionic Liquids from Hydroxycinnamic Acids as New Promising Bioactive Agents: A Combined Experimental and Theoretical Investigation. ACS Sustain. Chem. Eng. 2021, 9, 2975–2986. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K. A Comprehensive Review of Non-Steroidal Anti- Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Bessone, F. Non-Steroidal Anti-inflammatory Drugs: What Is the Actual Risk of Liver Damage? World J. Gastroenterol. 2010, 16, 5651–5661. [Google Scholar] [CrossRef]
- Lipinski, C.A. Poor Aqueous Solubility—An Industry Wide Problem in ADME Screening. Am. Pharm. Rev. 2002, 5, 82–85. [Google Scholar]
- Stolberg, V.B. Painkillers: History, Science, and Issues, 1st ed.; ABC-CLIO, Ed.; Greenwood: Santa Barbara, CA, USA, 2016. [Google Scholar]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse Effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs, Aspirin and Coxibs) on Upper Gastrointestinal Tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Arfaei, S. Selective COX-2 Inhibitors: A Review of Their Structure-Activity Relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar] [PubMed]
- Wu, H.; Deng, Z.; Zhou, B.; Qi, M.; Hong, M.; Ren, G. Improved transdermal permeability of ibuprofen by ionic liquid technology: Correlation between counterion structure and the physicochemical and biological properties. J. Mol. Liq. 2019, 283, 399–409. [Google Scholar] [CrossRef]
- Santos, M.M.; Raposo, L.R.; Carrera, G.V.S.M.; Costa, A.; Dionísio, M.; Baptista, P.V.; Fernandes, A.R.; Branco, L.C. Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug. Chem. Med. Chem. 2019, 14, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Stockerm, M.W.; Healy, A.M.; Ferguson, S. Spray Encapsulation as a Formulation Strategy for Drug-Based Room Temperature Ionic Liquids: Exploiting Drug–Polymer Immiscibility to Enable Processing for Solid Dosage Forms. Mol. Pharm. 2020, 17, 3412–3424. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Neves, B.M.; Se, G.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Design of Nonsteroidal Anti-Inflammatory Drug-Based Ionic Liquids with Improved Water Solubility and Drug Delivery. ACS Sustain. Chem. Eng. 2019, 7, 14126–14134. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic liquids with N-methyl-2-pyrrolidonium cation as an enhancer for topical drug delivery: Synthesis, characterization, and skin-penetration evaluation. J. Mol. Liq. 2020, 299, 167–7322. [Google Scholar] [CrossRef]
- Abednejad, A.; Ghaee, A.; Morais, E.S.; Sharma, M.; Neves, B.M.; Freire, M.G.; Nourmohammadi, J.; Mehrizi, A.A. Polyvinylidene fluoride–Hyaluronic acid wound dressing comprised of ionic liquids for controlled drug delivery and dual therapeutic behavior. Acta Biomater. 2019, 100, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Panić, J.J.; Tot, A.; Drid, P.; Gadžurić, S.; Vraneš, M. Design and analysis of interactions in ionic liquids based on procaine and pharmaceutically active anions. Eur. J. Pharm. Sci. 2021, 166, 105966. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chopade, S.A.; Guo, Y.; Early, J.T.; Tang, B.; Wang, E.; Hillmyer, M.A.; Lodge, T.P.; Sun, C.C. Preparation, Characterization, and Formulation Development of Drug–Drug Protic Ionic Liquids of Diphenhydramine with Ibuprofen and Naproxen. Mol. Pharm. 2018, 15, 4190–4201. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, C.P.; Wust, K.; Tier, A.Z.; Beck, T.S.; Rodrigues, L.V.; Vaucher, R.A.; Bolzan, L.P.; Terra, S.; Soares, F.; Martins, M.A.P. Novel ibuprofenate-and docusate-based ionic liquids: Emergence of antimicrobial activity. RSC Adv. 2016, 6, 100476–100486. [Google Scholar] [CrossRef]
- Wust, K.M.; Beck, T.S.; Burrow, R.A.; Oliveira, S.M.; Brum, E.S.; Brusco, I.; Machado, G.; Bianchi, O.; Villetti, M.A.; Frizzo, C.P. Physicochemical characterization, released profile, and antinociceptive activity of diphenhydraminium ibuprofenate supported on mesoporous silica. Mater. Sci. Eng. C. 2020, 108, 110194. [Google Scholar] [CrossRef]
- Janus, E.; Ossowicz, P.; Klebeko, J.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Klimowicz, A. Enhancement of ibuprofen solubility and skin permeation by conjugation with l-valine alkyl esters. RSC Adv. 2020, 10, 7570–7584. [Google Scholar] [CrossRef]
- Ossowicz-Rupniewska, P.; Klebeko, J.; Świątek, E.; Bilska, K.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Struk, Ł.; Wenelska, K.; Klimowicz, A.; et al. Influence of the Type of Amino Acid on the Permeability and Properties of Ibuprofenates of Isopropyl Amino Acid Esters. Int. J. Mol. Sci. 2022, 23, 4158. [Google Scholar] [CrossRef]
- Gaspar, M.M.; Calado, S.; Pereira, J.; Ferronha, H.; Correia, I.; Castro, H.; Tomás, A.M.; Cruz, M.E.M. Targeted delivery of paromomycin in murine infectious diseases through association to nano lipid systems. Nanomedicine 2015, 11, 1851–1860. [Google Scholar] [CrossRef]
- Mizushima, Y.; Kobayashi, M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmcol. 1968, 20, 169–173. [Google Scholar] [CrossRef]
- Kulmacz, R.J.; Lands, W.E.M. Requirements for Hydroperoxide by the Cyclooxygenase and Peroxidase Activities of Prostaglandin H Synthase. Prostaglandins 1983, 25, 531–540. [Google Scholar] [CrossRef]
- Araújo, J.M.M.; Ferreira, R.; Marrucho, I.M.; Rebelo, L.P.N. Solvation of Nucleobases in 1,3-Dialkylimidazolium Acetate Ionic Liquids: NMR Spectroscopy Insights into the Dissolution Mechanism. Phys. Chem. B. 2011, 115, 10739–10749. [Google Scholar] [CrossRef]
- Araújo, J.M.M.; Pereiro, A.B.; Lopes, J.N.C.; Rebelo, L.P.N.; Marrucho, I.M. Hydrogen-Bonding and the Dissolution Mechanism of Uracil in an Acetate Ionic Liquid: New Insights from NMR Spectroscopy and Quantum Chemical Calculations. J. Phys. Chem. B. 2013, 117, 4109–4120. [Google Scholar] [CrossRef]
- Ferreira, M.L.; Araújo, J.M.M.; Vega, L.F.; Llovell, F.; Pereiro, A.B. Functionalization of fluorinated ionic liquids: A combined experimental-theoretical study. J. Mol. Liq. 2020, 302, 112489. [Google Scholar] [CrossRef]
- Bica, K.; Rodríguez, H.; Gurau, G.; Cojocaru, O.A.; Riisager, A.; Fehrmannd, R.; Rogers, R.D. Pharmaceutically active ionic liquids with solids handling, enhanced thermal stability, and fast release. Chem. Commun. 2012, 48, 5422–5424. [Google Scholar] [CrossRef]
- Bustamante, P.; Pena, M.A.; Barra, J. The modified extended Hansen method to determine partial solubility parameters of drugs containing a single hydrogen bonding group and their sodium derivatives: Benzoic acid/Na and ibuprofen/Na. Int. J. Pharm. 2000, 194, 117–124. [Google Scholar] [CrossRef]
- Lu, C.; Cao, J.; Wanga, N.; Su, E. Significantly improving the solubility of non-steroidal anti-inflammatory drugs in deep eutectic solvents for potential non-aqueous liquid administration. Med. Chem. Commun. 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Viciosa, M.T.; Santos, G.; Costa, A.; Danède, F.; Branco, L.C.; Jordão, N.; Correia, N.T.; Dionísio, M. Dipolar motions and ionic conduction in an ibuprofen derived ionic liquid. Phys. Chem. Chem. Phys. 2015, 17, 24108–24120. [Google Scholar] [CrossRef]
- Dewland, P.M.; Reader, S.; Berry, P. Bioavailability of ibuprofen following oral administration of standard ibuprofen, sodium ibuprofen or ibuprofen acid incorporating poloxamer in healthy volunteers. BMC Clin. Pharmacol. 2009, 9, 19. [Google Scholar] [CrossRef]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the anticancer potential of limomene based therapeutic deep eutectic solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef]
- Egorova, K.S.; Seitkalieva, M.M.; Posvyatenkob, A.V.; Ananikov, V.P. An unexpected increase of toxicity of amino acid-containing ionic liquids. Toxicol. Res. 2015, 4, 152–159. [Google Scholar] [CrossRef]
- Dobler, D.; Schmidts, T.; Klingenhöfer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2013, 441, 620–627. [Google Scholar] [CrossRef]
- Hernández-Fernández, F.J.; Bayo, J.; Pérez de los Ríos, A.; Vicente, M.A.; Bernal, F.J.; Quesada-Medina, J. Discovering Less Toxic Ionic Liquids by Using the Microtox® Toxicity Test. Ecotoxicol. Environ. Saf. 2015, 116, 29–33. [Google Scholar] [CrossRef]
- Iyinagoro, C.; Nwankwo, N.; Ali, A.M.; Saba, R.; Kwatra, S.G.; Hussain, N.; Uzoka, C.C.; Prueksaritanond, S.; Mirrakhimov, A.E. Ibuprofen-induced hemolytic anemia. Case Rep. Hematol. 2013, 2013, 142865. [Google Scholar]
- Garratty, G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010, 24, 143–150. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Strategies to Identify and Mitigate Risks for First-in-Human and Early Clinical Trials with Investigational Medicinal Products; EMEA/CHMP/SWP/28367/07 Rev. 1; Committee for Medicinal Products for Human Use (CHMP): Amsterdam, The Netherlands, 2017. [Google Scholar]
- Orhan, H.; Sahi’n, G. In vitro effects of NSAIDS and paracetamol on oxidative stress-related parameters of human erythrocytes. Exp. Toxic. Pathol. 2001, 53, 133–140. [Google Scholar] [CrossRef]
- Vieira, N.S.; Oliveira, A.L.; Araújo, J.M.M.; Gaspar, M.M.; Pereiro, A.B. Ecotoxicity and Hemolytic Activity of Fluorinated Ionic Liquids. Sustain. Chem. 2021, 2, 115–126. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef]
- Sangeetha, G.; Vidhya, R. In vitro anti-inflammatory activity of different parts of Pedalium murex (L.). Inflammation 2016, 4, 31–36. [Google Scholar]
- Douadi, K.; Chafaa, S.; Douadi, T.; Al-Noaimi, M.; Kaabi, I. Azoimine quinoline derivatives: Synthesis, classical and electrochemical evaluation of antioxidant, anti-inflammatory, antimicrobial activities and the DNA/BSA binding. J. Mol. Struct. 2020, 1217, 128305. [Google Scholar] [CrossRef]
- Kumar, A.; Rani, A.; Venkatesu, P. A comparative study of the effects of the Hofmeister series anions of the ionic salts and ionic liquids on the stability of α-chymotrypsin. New J. Chem. 2015, 39, 938–952. [Google Scholar] [CrossRef]
- Bisht, M.; Venkatesu, P. Influence of cholinium-based ionic liquids on the structural stability and activity of α-chymotrypsin. New J.Chem. 2017, 41, 13902. [Google Scholar] [CrossRef]
- Lange, C.; Patil, G.; Rudolph, R. Ionic liquids as refolding additives: N’-alkyl and N’-(ω-hydroxyalkyl) N-methylimidazolium chlorides. Protein Sci. 2005, 14, 2693–2701. [Google Scholar] [CrossRef]
- Moelbert, S.; Normand, B.; De Los Rios, P. Kosmotropes and chaotropes: Modelling preferential exclusion, binding and aggregate stability. Biophys. Chem. 2004, 112, 45–57. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of hydrogen bonds to protein stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- Nugteren, D.H.; Hazelhof, E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim. Biophys. Acta Lipids Lipid Metab. 1973, 326, 448–461. [Google Scholar] [CrossRef]
- Hamberg, M.; Samuelsson, B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc. Natl. Acad. Sci. USA 1973, 70, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.L.; Chipman, J.G.; Robertson, D.L.; Erikson, R.L.; Simmons, D.L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl. Acad. Sci. USA 1991, 88, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Tacconelli, S.; Bruno, A.; Sostres, C.; Lanas, A. Managing the adverse effects of nonsteroidal anti-inflammatory drugs. Expert. Rev. Clin. Pharmacol. 2011, 4, 605–621. [Google Scholar] [CrossRef]
- Hutchison, R. COX-2-selective NSAIDs. Am. J. Nurs. Sci. 2004, 10, 52–56. [Google Scholar] [CrossRef]
- Simon, L.S.; Weaver, A.L.; Graham, D.Y.; Kivitz, A.J.; Lipsky, P.E.; Hubbard, R.C.; Isakson, P.C.; Verburg, K.M.; Yu, S.S.; Zhao, W.W.; et al. Anti-inflammatory and Upper Gastrointestinal Effects of Celecoxib in Rheumatoid Arthritis: A Randomized Controlled Trial. JAMA. 1999, 282, 1921–1928. [Google Scholar] [CrossRef]
- Brune, K.; Patrignani, P. New insights into the use of currently available non-steroidal anti-inflammatory drugs. J. Pain Res. 2015, 8, 105–118. [Google Scholar] [CrossRef]
- Bruno, A.; Tacconelli, S.; Patrignani, P. Variability in the response to non-steroidal anti-inflammatory drugs: Mechanisms and perspectives. BCPT 2014, 114, 56–63. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ibuprofen pathways. Pharm. Genom. 2015, 25, 96. [Google Scholar] [CrossRef]




| Chemical Structure | Designation and Acronym | Mw (g/mol) |
|---|---|---|
 | 2-(4-Isobutylphenyl) propionic acid Ibuprofen (Ibu) | 206.29 |
 | Sodium 2-(4-isobutylphenyl) propanoate Sodium ibuprofen salt (Na[Ibu]) | 228.26 |
 | 1-Ethyl-3-methylimidazolium Ibuprofenate [C2C1Im][Ibu] | 316.44 |
 | 1-(2-Hydroxyethyl)-3-methylimidazolium Ibuprofenate [C2(OH)C1Im][Ibu] | 332.44 |
 | Cholinium Ibuprofenate [N1112(OH)][Ibu] | 309.44 |
| Compounds | H2 (δ/ppm) | H3 (δ/ppm) |
|---|---|---|
| Ibuprofen | 3.63 | 1.35 |
| Na[Ibu] | 3.21 | 1.22 |
| [C2C1Im][Ibu] | 3.15 | 1.19 |
| [C2(OH)C1Im][Ibu] | 3.18 | 1.19 |
| [N1112(OH)][Ibu] | 3.18 | 1.20 |
 | ||
| Compound | Tg (°C) | Tm (°C) |
|---|---|---|
| Ibuprofen | −43.57 | 74.89 |
| [C2C1Im][Ibu] | −30.55 | 72.44 |
| [C2(OH)C1Im][Ibu] | – | −13.97 |
| [N1112(OH)][Ibu] | – | 70.89 |
| Compound | Solubility (mM) at 25 °C | |||
|---|---|---|---|---|
| Water | 0.15 M NaCl | pH 1.0 | pH 6.8 | |
| Ibuprofen | 0.2791 ± 0.0068 (0.3259 [23], 0.3394 [43]) | 0.3530 ± 0.0131 | 0.2146 ± 0.0053 | 20.21 ± 0.58 |
| Sodium ibuprofen | 1762 ± 52 | 1506 ± 30 | 1173 ± 57 | 1907 ± 11 |
| [N1112(OH)][Ibu] | 6783 ± 226 | 2519 ± 45 | 3159 ± 84 | 11,074 ± 438 |
| [C2C1Im][Ibu] | 7817 ± 160 | - † | - † | - † |
| [C2(OH)C1Im][Ibu] | 7426 ± 357 | - † | - † | - † |
| Compound | EC50 (mM) |
|---|---|
| Ibuprofen | 4.052 ± 0.010 (2.893 ± 0.059 [46]) |
| [C2C1Im][Ibu] | 5.683 ± 0.001 |
| [C2(OH)C1Im][Ibu] | 5.523 ± 0.001 |
| [N1112(OH)][Ibu] | 5.508 ± 0.001 |
| [C2C1Im]Cl | 70.66 ± 0.011 (32.10 ± 1.84 [47]) |
| [C2(OH)C1Im]Cl | 137.5 ± 0.001 |
| [N1112(OH)]Cl | 178.1 ± 0.001 |
| Compound | Inhibition of COX-1 (Ovine) (%) | Inhibition of COX-2 (Human) (%) | COX-1/COX-2 Selectivity |
|---|---|---|---|
| Ibuprofen | 56.18 ± 2.60 | 65.13 ± 4.25 | 0.863 ± 0.069 |
| [C2C1Im][Ibu] | 51.50 ± 1.98 | 65.13 ± 4.27 | 0.791 ± 0.060 |
| [C2(OH)C1Im][Ibu] | 56.35 ± 4.35 | 74.04 ± 4.42 | 0.761 ± 0.074 |
| [N1112(OH)][Ibu] | 49.48 ± 4.64 | 73.07 ± 3.55 | 0.677 ± 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, J.C.; Vieira, N.S.M.; Gaspar, M.M.; Pereiro, A.B.; Araújo, J.M.M. Human Cytotoxicity, Hemolytic Activity, Anti-Inflammatory Activity and Aqueous Solubility of Ibuprofen-Based Ionic Liquids. Sustain. Chem. 2022, 3, 358-375. https://0-doi-org.brum.beds.ac.uk/10.3390/suschem3030023
Bastos JC, Vieira NSM, Gaspar MM, Pereiro AB, Araújo JMM. Human Cytotoxicity, Hemolytic Activity, Anti-Inflammatory Activity and Aqueous Solubility of Ibuprofen-Based Ionic Liquids. Sustainable Chemistry. 2022; 3(3):358-375. https://0-doi-org.brum.beds.ac.uk/10.3390/suschem3030023
Chicago/Turabian StyleBastos, Joana C., Nicole S. M. Vieira, Maria Manuela Gaspar, Ana B. Pereiro, and João M. M. Araújo. 2022. "Human Cytotoxicity, Hemolytic Activity, Anti-Inflammatory Activity and Aqueous Solubility of Ibuprofen-Based Ionic Liquids" Sustainable Chemistry 3, no. 3: 358-375. https://0-doi-org.brum.beds.ac.uk/10.3390/suschem3030023