Lower Levels of Vestibular Developmental Stability in Slow-Moving than Fast-Moving Primates
Abstract
:1. Introduction
- Do slow-moving species show higher levels of SCC morphological variation than fast-moving taxa? Is stabilising selection stronger in animals with rapid postural adjustments and less restrictive in species with slower locomotor repertoires?
- If a selective difference exists, does it affect fluctuating asymmetry levels expressed in SCC morphology, and is the level of asymmetry higher in slow-moving taxa than in fast-moving ones? How strong are the developmental stabilising forces during the early stages of ontogeny?
2. Materials and Methods
2.1. Specimens and Selected Taxa
2.2. 3D Data Acquisition
2.3. Semilandmarks Protocol
2.4. Intraspecific Variation in Orientation and Size of the Semicircular Canals
2.5. Semicircular Canal Shape and Size Variation
2.6. Vestibular Fluctuating Asymmetry
3. Results
3.1. Intraspecific Variation in Orientation and Size of the Semicircular Canals
3.1.1. Angular Values
3.1.2. SCC Size and Common Crus Length
3.2. Semicircular Canal Shape Variation
3.2.1. Interspecific Patterns of Shape Variation Revealed by PCA
3.2.2. Intraspecific Patterns of Shape Variation
3.3. Semicircular Canal System FA Levels
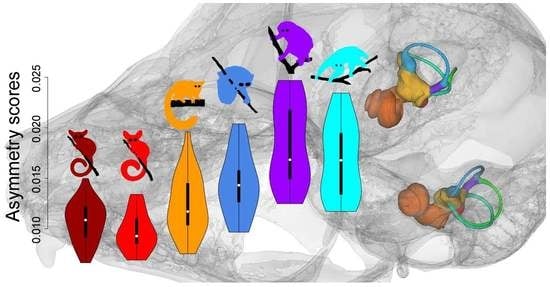
3.3.1. Asymmetry Scores and FA10 Levels
3.3.2. Shared Patterns of FA Shape Variation
3.3.3. Intraspecific Patterns of FA Shape Variation
4. Discussion
4.1. Interspecific and Intraspecific Semicircular Canal Morphological Variation
4.2. Orthogonality and Angular Variance
4.3. Coplanarity of Synergistic Canal Pairs
4.4. Semicircular Canal Phenotypic Variation and Canalisation Mechanisms
4.5. Semicircular Canal Symmetry
4.6. Canalisation and Developmental Stability
4.7. Heritability
5. Conclusions
5.1. Prenatal Developmental Timing of the Semicircular Canal System
5.2. Postnatal Relative Position and Orientation of Labyrinths
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodrich, E.S. Studies on the Structure and Development of Vertebrates; Macmillan: London, UK, 1930; ISBN 0-226-30354-3. [Google Scholar]
- Welgampola, M.S.; Colebatch, J.G. Vestibulocollic Reflexes: Normal Values and the Effect of Age. Clin. Neurophysiol. 2001, 112, 1971–1979. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Cullen, K.E. Vestibular Control of the Head: Possible Functions of the Vestibulocollic Reflex. Exp. Brain Res. 2011, 210, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Bar-Oz, G.; Dayan, T. FOCUS: On the Use of the Petrous Bone for Estimating Cranial Abundance in Fossil Assemblages. J. Archaeol. Sci. 2007, 34, 1356–1360. [Google Scholar] [CrossRef]
- Zonneveld, F.; Spoor, C.F.; Wind, J. The Use of CT in the Study of the Internal Morphology of Hominid Fossils. Medicamundi 1989, 34, 117–128. [Google Scholar]
- Spoor, C.F. The Comparative Morphology and Phylogeny of the Human Bony Labyrinth. Ph.D. Dissertation, Utrecht University, Utrecht, The Netherlands, 1993. [Google Scholar]
- Spoor, C.F.; Wood, B.; Zonneveld, F. Implications of Early Hominid Labyrinthine Morphology for Evolution of Human Bipedal Locomotion. Nature 1994, 369, 645–648. [Google Scholar] [CrossRef]
- Spoor, C.F.; Wood, B.; Zonneveld, F. Evidence for a Link between Human Semicircular Canal Size and Bipedal Behaviour. J. Hum. Evol. 1996, 30, 183–187. [Google Scholar] [CrossRef]
- Spoor, C.F.; Zonneveld, F. Morphometry of the Primate Bony Labyrinth: A New Method Based on High-Resolution Computed Tomography. J. Anat. 1995, 186, 271–286. [Google Scholar]
- Spoor, F.; Zonneveld, F. Comparative Review of the Human Bony Labyrinth. Am. J. Phys. Anthropol. 1998, 107, 211–251. [Google Scholar] [CrossRef]
- Spoor, F.; Garland, T.; Krovitz, G.; Ryan, T.M.; Silcox, M.T.; Walker, A. The Primate Semicircular Canal System and Locomotion. Proc. Natl. Acad. Sci. USA 2007, 104, 10808–10812. [Google Scholar] [CrossRef] [Green Version]
- Walker, A.; Ryan, T.M.; Silcox, M.T.; Simons, E.L.; Spoor, F. The Semicircular Canal System and Locomotion: The Case of Extinct Lemuroids and Lorisoids. Evol. Anthropol. Issues News Rev. 2008, 17, 135–145. [Google Scholar] [CrossRef]
- Silcox, M.T.; Bloch, J.I.; Boyer, D.M.; Godinot, M.; Ryan, T.M.; Spoor, F.; Walker, A. Semicircular Canal System in Early Primates. J. Hum. Evol. 2009, 56, 315–327. [Google Scholar] [CrossRef]
- Ryan, T.M.; Silcox, M.T.; Walker, A.; Mao, X.; Begun, D.R.; Benefit, B.R.; Gingerich, P.D.; Köhler, M.; Kordos, L.; McCrossin, M.L.; et al. Evolution of Locomotion in Anthropoidea: The Semicircular Canal Evidence. Proc. R. Soc. B Biol. Sci. 2012, 279, 3467–3475. [Google Scholar] [CrossRef] [Green Version]
- Bernardi, M.; Couette, S. Eocene Paleoecology of Adapis parisiensis (Primate, Adapidae): From Inner Ear to Lifestyle. Anat. Rec. 2017, 300, 1576–1588. [Google Scholar] [CrossRef] [Green Version]
- Berlin, J.C.; Kirk, E.C.; Rowe, T.B. Functional Implications of Ubiquitous Semicircular Canal Non-Orthogonality in Mammals. PLoS ONE 2013, 8, e79585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spoor, F.; Bajpai, S.; Hussain, S.T.; Kumar, K.; Thewissen, J.G.M. Vestibular Evidence for the Evolution of Aquatic Behaviour in Early Cetaceans. Nature 2002, 417, 163–166. [Google Scholar] [CrossRef]
- Orliac, M.J.; Benoit, J.; O’Leary, M.A. The Inner Ear of Diacodexis, the Oldest Artiodactyl Mammal. J. Anat. 2012, 221, 417–426. [Google Scholar] [CrossRef]
- Alloing-Séguier, L.; Sánchez-Villagra, M.R.; Lee, M.S.Y.; Lebrun, R. The Bony Labyrinth in Diprotodontian Marsupial Mammals: Diversity in Extant and Extinct Forms and Relationships with Size and Phylogeny. J. Mamm. Evol. 2013, 20, 191–198. [Google Scholar] [CrossRef]
- Ravel, A.; Orliac, M.J. The Inner Ear Morphology of the ‘Condylarthran’ Hyopsodus lepidus. Hist. Biol. 2015, 27, 957–969. [Google Scholar] [CrossRef]
- Pfaff, C.; Martin, T.; Ruf, I. Bony Labyrinth Morphometry Indicates Locomotor Adaptations in the Squirrel-Related Clade (Rodentia, Mammalia). Proc. R. Soc. B Biol. Sci. 2015, 282, 20150744. [Google Scholar] [CrossRef] [Green Version]
- Grohé, C.; Tseng, Z.J.; Lebrun, R.; Boistel, R.; Flynn, J.J. Bony Labyrinth Shape Variation in Extant Carnivora: A Case Study of Musteloidea. J. Anat. 2016, 228, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, C.; Czerny, S.; Nagel, D.; Kriwet, J. Functional Morphological Adaptations of the Bony Labyrinth in Marsupials (Mammalia, Theria). J. Morphol. 2017, 278, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Hullar, T.E.; Williams, C.D. Geometry of the Semicircular Canals of the Chinchilla (Chinchilla laniger). Hear. Res. 2006, 213, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, D.R.; Hullar, T.E. Planar Relationships of the Semicircular Canals in Two Strains of Mice. J. Assoc. Res. Otolaryngol. 2006, 7, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, P.G.; Jeffery, N. Semicircular Canals and Agility: The Influence of Size and Shape Measures. J. Anat. 2010, 216, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Malinzak, M.D.; Kay, R.F.; Hullar, T.E. Locomotor Head Movements and Semicircular Canal Morphology in Primates. Proc. Natl. Acad. Sci. USA 2012, 109, 17914–17919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graf, W. Motion Detection in Physical Space and Its Peripheral and Central Representation. Ann. N. Y. Acad. Sci. 1988, 545, 154–169. [Google Scholar] [CrossRef]
- Perier, A.; Lebrun, R.; Marivaux, L. Different Level of Intraspecific Variation of the Bony Labyrinth Morphology in Slow- versus Fast-Moving Primates. J. Mamm. Evol. 2016, 23, 353–368. [Google Scholar] [CrossRef]
- Bhagat, R.; Bertrand, O.; Silcox, M.T. Evolution of Arboreality and Fossoriality in Squirrels and Aplodontid Rodents: Insights from the Semicircular Canals of Fossil Rodents. J. Anat. 2020, 238, 96–112. [Google Scholar] [CrossRef]
- Billet, G.; Hautier, L.; Asher, R.J.; Schwarz, C.; Crumpton, N.; Martin, T.; Ruf, I. High Morphological Variation of Vestibular System Accompanies Slow and Infrequent Locomotion in Three-Toed Sloths. Proc. R. Soc. B Biol. Sci. 2012, 279, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, L.A.; Malinzak, M.D.; Kay, R.F. Intraspecific Variation in Semicircular Canal Morphology—A Missing Element in Adaptive Scenarios? Am. J. Phys. Anthropol. 2019, 168, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Waddington, C.H. Canalization of Development and the Inheritance of Acquired Characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- Rodgers, J.C. Comparative Morphology of the Vestibular Semicircular Canals in Therian Mammals. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2011. [Google Scholar]
- Welker, K.L.; Orkin, J.D.; Ryan, T.M. Analysis of Intraindividual and Intraspecific Variation in Semicircular Canal Dimensions Using High-Resolution x-Ray Computed Tomography. J. Anat. 2009, 215, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.P. Analyzing Fluctuating Asymmetry with Geometric Morphometrics: Concepts, Methods, and Applications. Symmetry 2015, 7, 843–934. [Google Scholar] [CrossRef] [Green Version]
- Van Valen, L. A Study of Fluctuating Asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Debat, V.; Peronnet, F. Asymmetric Flies: The Control of Developmental Noise in Drosophila. Fly (Austin) 2013, 7, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Parsons, P.A. Fluctuating Asymmetry: A Biological Monitor of Environmental and Genomic Stress. Heredity 1992, 68, 361–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, G.M. Fluctuating Asymmetry: A Technique for Measuring Developmental Stress of Genetic and Environmental Origin. Acta Zool. Fenn. 1992, 191, 31–35. [Google Scholar]
- Møller, A.P. Female Swallow Preference for Symmetrical Male Sexual Ornaments. Nature 1992, 357, 238–240. [Google Scholar] [CrossRef]
- Møller, A.P. Fluctuating Asymmetry in Male Sexual Ornaments May Reliably Reveal Male Quality. Anim. Behav. 1990, 40, 1185–1187. [Google Scholar] [CrossRef]
- Clarke, G.M. Developmental Stability and Fitness: The Evidence Is Not Quite so Clear. Am. Nat. 1998, 152, 762–766. [Google Scholar] [CrossRef]
- David, P. Heterozygosity–Fitness Correlations: New Perspectives on Old Problems. Heredity 1998, 80, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Crespi, B.J.; Vanderkist, B.A. Fluctuating Asymmetry in Vestigial and Functional Traits of a Haplodiploid Insect. Heredity 1997, 79, 624–630. [Google Scholar] [CrossRef]
- Garnier, S.; Gidaszewski, N.; Charlot, M.; Rasplus, J.-Y.; Alibert, P. Hybridization, Developmental Stability, and Functionality of Morphological Traits in the Ground Beetle Carabus solieri (Coleoptera, Carabidae). Biol. J. Linn. Soc. 2006, 89, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Ashton, E.H.; Oxnard, C.E. Locomotor Patterns in Primates. Proc. Zool. Soc. Lond. 1964, 142, 1–28. [Google Scholar] [CrossRef]
- Napier, J.R.; Napier, P.H. A Handbook of Living Primates.; Academic Press: London, UK, 1967. [Google Scholar]
- Martin, R.D. Primate Origins and Evolution. A Phylogenetic Reconstruction; Chapman and Hall: London, UK, 1990; ISBN 0-691-08565-X. [Google Scholar]
- Hunt, K.D.; Cant, J.G.H.; Gebo, D.L.; Rose, M.D.; Walker, S.E.; Youlatos, D. Standardized Descriptions of Primate Locomotor and Postural Modes. Primates 1996, 37, 363–387. [Google Scholar] [CrossRef]
- Boyer, D.M.; Gunnell, G.F.; Kaufman, S.; McGeary, T.M. MorphoSource: Archiving and Sharing 3-D Digital Specimen Data. Paleontol. Soc. Pap. 2016, 22, 157–181. [Google Scholar] [CrossRef]
- Walker, A. Locomotor adaptations in past and present prosimian primates. In Primate Locomotion; Jenkins, F.A., Ed.; Academic Press: New York, NY, USA; London, UK, 1974; pp. 349–382. [Google Scholar]
- Masters, J. Sluggards and drunkards? In Evolution, Ecology and Conservation of Lorises and Pottos; Nekaris, K.A.I., Burrows, A.M., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 19–32. ISBN 978-1-108-67652-6. [Google Scholar]
- Chen, J.-H.; Pan, D.; Groves, C.; Wang, Y.-X.; Narushima, E.; Fitch-Snyder, H.; Crow, P.; Thanh, V.N.; Ryder, O.; Zhang, H.-W.; et al. Molecular Phylogeny of Nycticebus Inferred from Mitochondrial Genes. Int. J. Primatol. 2006, 27, 1187–1200. [Google Scholar] [CrossRef]
- Groves, C. Primate Taxonomy; Smithsonian Institution Press: Washington, DC, USA, 2001; ISBN 1-56098-872-X. [Google Scholar]
- Groves, C. Systematics of the genus Nycticebus. In Proceeding of the Third International Congress of Primatology, Zurich 1970, Vol. 1: Taxonomy, Anatomy, Reproduction; Biegert, J., Leutenegger, W., Eds.; Karger: Basel, Switzerland, 1971; Volume 1, pp. 44–53. [Google Scholar]
- Jenkins, P.D. Catalogue of Primates in the British Museum (Natural History) and Elsewhere in the British Isles. Part IV: Suborder Strepsirrhini, Including the Subfossil Madagsacan Lemurs and the Family Tarsiidae.; British Museum: London, UK, 1987. [Google Scholar]
- Mittermeier, R.A.; Rylands, A.B.; Wilson, D.E. Handbook of the Mammals of the World. Volume 3. Primates; Lynx Edicions: Barcelona, Spain, 2013. [Google Scholar]
- Butynski, T.; de Jong, Y. Distribution of the Potto Perodicticus potto (Primates: Lorisidae) in Eastern Africa, with a Description of a New Subspecies from Mount Kenya. J. East Afr. Nat. Hist. 2007, 96, 113–147. [Google Scholar] [CrossRef]
- Oates, J.F. Primates of West Africa: A Field Guide and Natural History; Conservation International: Arlington, Virginia, 2011; ISBN 1-934151-48-3. [Google Scholar]
- Nekaris, K.A.I. Family Lorisidae (angwantibos, pottos and lorises). In Handbook of the Mammals of the World. Volume 3. Primates; Mittermeier, R.A., Rylands, A.B., Wilson, D.E., Eds.; Lynx Edicions: Barcelona, Spain, 2013; pp. 210–235. [Google Scholar]
- Forbes, H.O. A Handbook to the Primates, Vol. I.; Bowdler Sharpe, R., Ed.; Allen’s Naturalist’s Library: London, UK, 1896. [Google Scholar]
- Walker, A. The Locomotion of the Lorises, with Special Reference to the Potto. Afr. J. Ecol. 1969, 7, 1–5. [Google Scholar] [CrossRef]
- Subramoniam, S. Some Observations on the Habits of the Slender Loris, Loris tardigradus (Linnaeus). J. Bombay Nat. Hist. Soc. 1957, 54, 387–398. [Google Scholar]
- Walker, A. Prosimian locomotor behavior. In The Study of Prosimian Behavior; Doyle, G.A., Martin, R.D., Eds.; Academic Press: Cambridge, UK, 1979; pp. 543–565. ISBN 978-0-12-222150-7. [Google Scholar]
- Gamage, S.; Groves, C.; Marikar, F.M.M.T.; Turner, C.; Padmalal, K.U.K.G.; Kotagama, S. The Taxonomy, Distribution, and Conservation Status of the Slender Loris (Primates, Lorisidae: Loris) in Sri Lanka. Primate Conserv. 2017, 31, 83–106. [Google Scholar]
- Oxnard, C.E.; Crompton, R.H.; Lieberman, S.S. Animal Lifestyles and Anatomies: The Case of the Prosimian Primates; University of Washington Press: Washington, DC, USA, 1990; ISBN 0-295-96839-7. [Google Scholar]
- Demes, B.; Jungers, W.L.; Nieschalk, U. Size and speed-related aspects of quadrupedal walking in slender and slow lorises. In Gravity, Posture and Locomotion in Primates; Jouffroy, F.K., Stack, M.H., Niemitz, C., Eds.; Il Sedicesimo: Firenze, Italy, 1990; pp. 175–197. [Google Scholar]
- Nekaris, K.A.I. Activity Budget and Positional Behavior of the Mysore Slender Loris (Loris tardigradus lydekkerianus): Implications for Slow Climbing Locomotion. Folia Primatol. 2001, 72, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.I.; Stevens, N.J. Not All Lorises Are Slow: Rapid Arboreal Locomotion in Loris tardigradus of Southwestern Sri Lanka. Am. J. Primatol. 2007, 69, 113–121. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Bearder, S.K. The strepsirrhine primates of Asia and mainland Africa: Diversity shrouded in darkness. In Primates in perspective; Campbell, C., Fuentes, A., MacKinnon, K., Stumpf, R., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 34–54. [Google Scholar]
- Günther, M.M.; Ishida, H.; Kumakura, H.; Nakano, Y. The Jump as a Fast Mode of Locomotion in Arboreal and Terrestrial Biotopes. Z. Für Morphol. Anthropol. 1991, 78, 341–372. [Google Scholar] [CrossRef]
- Crompton, R.H.; Sellers, W.I. A consideration of leaping locomotion as a means of predator avoidance in prosimian primates. In Primate Anti-Predator Strategies; Gursky, S.L., Nekaris, K.A.I., Eds.; Springer: Boston, MA, USA, 2007; pp. 127–145. ISBN 978-0-387-34810-0. [Google Scholar]
- Crompton, R.H.; Sellers, W.I.; Günther, M.M. Energetic Efficiency and Ecology as Selective Factors in the Saltatory Adaptation of Prosimian Primates. Proc. R. Soc. Lond. B Biol. Sci. 1993, 254, 41–45. [Google Scholar] [CrossRef]
- Bearder, S.K. Lorises, bushbabies, and tarsiers: Diverse societies in solitary foragers. In Primate Societies; Smuts, B.B., Cheney, D.L., Seyfarth, R.M., Wrangham, R.W., Eds.; University of Chicago Press: Chicago, IL, USA, 2008; pp. 11–24. ISBN 978-0-226-22046-8. [Google Scholar]
- Génin, F.; Yokwana, A.; Kom, N.; Couette, S.; Dieuleveut, T.; Nash, S.D.; Masters, J.C. A New Galago Species for South Africa (Primates: Strepsirhini: Galagidae). Afr. Zool. 2016, 51, 135–143. [Google Scholar] [CrossRef]
- Masters, J.C.; Génin, F.; Couette, S.; Groves, C.P.; Nash, S.D.; Delpero, M.; Pozzi, L. A New Genus for the Eastern Dwarf Galagos (Primates: Galagidae). Zool. J. Linn. Soc. 2017, 181, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Lösel, P.D.; van de Kamp, T.; Jayme, A.; Ershov, A.; Faragó, T.; Pichler, O.; Tan Jerome, N.; Aadepu, N.; Bremer, S.; Chilingaryan, S.A.; et al. Introducing Biomedisa as an Open-Source Online Platform for Biomedical Image Segmentation. Nat. Commun. 2020, 11, 5577. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, R. MorphoDig, an Open-Source 3D Freeware Dedicated to Biology. In Proceedings of the IPC5 The 5th International Palaeontological Congress, Paris, France, 9–13 July 2018. [Google Scholar]
- Benoit, J.; Legendre, L.J.; Farke, A.A.; Neenan, J.M.; Mennecart, B.; Costeur, L.; Merigeaud, S.; Manger, P.R. A Test of the Lateral Semicircular Canal Correlation to Head Posture, Diet and Other Biological Traits in “Ungulate” Mammals. Sci. Rep. 2020, 10, 1–22. [Google Scholar] [CrossRef]
- Adams, D.; Collyer, M.; Kaliontzopoulou, A.; Baken, E. Geomorph: Software for Geometric Morphometric Analyses, R Package Version 4.0; 2021; Available online: https://cran.r-project.org/web/packages/geomorph/index.html (accessed on 15 November 2021).
- Yezerinac, S.M.; Lougheed, S.C.; Handford, P. Measurement Error and Morphometric Studies: Statistical Power and Observer Experience. Syst. Biol. 1992, 41, 471–482. [Google Scholar] [CrossRef]
- Palmer, A.R. Fluctuating asymmetry analyses: A primer. In Developmental Instability: Its Origins and Evolutionary Implications; Markow, T.A., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 335–364. ISBN 978-94-010-4357-1. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021.
- Team RStudio. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2021. [Google Scholar]
- Schlager, S. Chapter 9—Morpho and Rvcg—Shape analysis in R: R-packages for geometric morphometrics, shape analysis and surface manipulations. In Statistical Shape and Deformation Analysis; Zheng, G., Li, S., Székely, G., Eds.; Academic Press: Cambridge, UK, 2017; pp. 217–256. ISBN 978-0-12-810493-4. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. 12—Disparity and variation. In Geometric Morphometrics for Biologists; Zelditch, M.L., Swiderski, D.L., Sheets, H.D., Fink, W.L., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 293–319. ISBN 978-0-12-778460-1. [Google Scholar]
- Webster, M.; Sheets, H.D. A Practical Introduction to Landmark-Based Geometric Morphometrics. Paleontol. Soc. Pap. 2010, 16, 163–188. [Google Scholar] [CrossRef] [Green Version]
- Matano, S.; Kubo, T.; Günther, M.M. Semicircular Canal Organ in Three Primate Species and Behavioural Correlations. Fortschr Zool 1985, 30, 677–680. [Google Scholar]
- David, R.; Droulez, J.; Allain, R.; Berthoz, A.; Janvier, P.; Bennequin, D. Motion from the Past. A New Method to Infer Vestibular Capacities of Extinct Species. Comptes Rendus Palevol 2010, 9, 397–410. [Google Scholar] [CrossRef]
- Cerio, D.G.; Witmer, L.M. Intraspecific Variation and Symmetry of the Inner-Ear Labyrinth in a Population of Wild Turkeys: Implications for Paleontological Reconstructions. PeerJ 2019, 7, e7355. [Google Scholar] [CrossRef]
- Ward, D.L.; Schroeder, L.; Pomeroy, E.; Roy, J.E.; Buck, L.T.; Stock, J.T.; Martin-Gronert, M.; Ozanne, S.E.; Silcox, M.T.; Viola, T.B. Early Life Malnutrition and Fluctuating Asymmetry in the Rat Bony Labyrinth. Anat. Rec. 2021, 304, 2645–2660. [Google Scholar] [CrossRef]
- Lebrun, R.; León, M.P.D.; Tafforeau, P.; Zollikofer, C. Deep Evolutionary Roots of Strepsirrhine Primate Labyrinthine Morphology. J. Anat. 2010, 216, 368–380. [Google Scholar] [CrossRef]
- de León, M.S.P.; Koesbardiati, T.; Weissmann, J.D.; Milella, M.; Reyna-Blanco, C.S.; Suwa, G.; Kondo, O.; Malaspinas, A.-S.; White, T.D.; Zollikofer, C.P.E. Human Bony Labyrinth Is an Indicator of Population History and Dispersal from Africa. Proc. Natl. Acad. Sci. USA 2018, 115, 4128–4133. [Google Scholar] [CrossRef] [Green Version]
- Beaudet, A.; Clarke, R.J.; Bruxelles, L.; Carlson, K.J.; Crompton, R.; de Beer, F.; Dhaene, J.; Heaton, J.L.; Jakata, K.; Jashashvili, T.; et al. The Bony Labyrinth of StW 573 (“Little Foot”): Implications for Early Hominin Evolution and Paleobiology. J. Hum. Evol. 2019, 127, 67–80. [Google Scholar] [CrossRef]
- Urciuoli, A.; Zanolli, C.; Beaudet, A.; Dumoncel, J.; Santos, F.; Moyà-Solà, S.; Alba, D.M. The Evolution of the Vestibular Apparatus in Apes and Humans. eLife 2020, 9, e51261. [Google Scholar] [CrossRef] [PubMed]
- Gunz, P.; Ramsier, M.; Kuhrig, M.; Hublin, J.-J.; Spoor, F. The Mammalian Bony Labyrinth Reconsidered, Introducing a Comprehensive Geometric Morphometric Approach. J. Anat. 2012, 220, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.A.; Kriwet, J.; Weber, G.W.; Pfaff, C. Carnivoran Hunting Style and Phylogeny Reflected in Bony Labyrinth Morphometry. Sci. Rep. 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Hullar, T.E. Relationship of Semicircular Canal Size to Vestibular-Nerve Afferent Sensitivity in Mammals. J. Neurophysiol. 2007, 98, 3197–3205. [Google Scholar] [CrossRef] [Green Version]
- Graf, W.; Klam, F. Le système vestibulaire: Anatomie fonctionnelle et comparée, évolution et développement. Comptes Rendus Palevol 2006, 5, 637–655. [Google Scholar] [CrossRef]
- Debat, V.; David, P. Mapping Phenotypes: Canalization, Plasticity and Developmental Stability. Trends Ecol. Evol. 2001, 16, 555–561. [Google Scholar] [CrossRef]
- Hoffmann, A.; Woods, R. Trait Variability and Stress: Canalization, Developmental Stability and the Need for a Broad Approach. Ecol. Lett. 2001, 4, 97–101. [Google Scholar] [CrossRef]
- Réale, D.; Roff, D.A. Inbreeding, Developmental Stability, and Canalization in the Sand Cricket Gryllus firmus. Evolution 2003, 57, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Nekaris, K.A.-I.; Starr, C.R.; Collins, R.L.; Wilson, A. Comparative ecology of exudate feeding by lorises (Nycticebus, Loris) and pottos (Perodicticus, Arctocebus). In The Evolution of Exudativory in Primates; Burrows, A.M., Nash, L.T., Eds.; Developments in Primatology: Progress and Prospects; Springer: New York, NY, USA, 2010; pp. 155–168. ISBN 978-1-4419-6661-2. [Google Scholar]
- Graham, J.H.; Raz, S.; Hel-Or, H.; Nevo, E. Fluctuating Asymmetry: Methods, Theory, and Applications. Symmetry 2010, 2, 466–540. [Google Scholar] [CrossRef] [Green Version]
- Debat, V.; Bloyer, S.; Faradji, F.; Gidaszewski, N.; Navarro, N.; Orozco-terWengel, P.; Ribeiro, V.; Schlötterer, C.; Deutsch, J.S.; Peronnet, F. Developmental Stability: A Major Role for Cyclin G in Drosophila melanogaster. PLoS Genet. 2011, 7, e1002314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallgrímsson, B.; Willmore, K.; Hall, B.K. Canalization, Developmental Stability, and Morphological Integration in Primate Limbs. Am. J. Phys. Anthropol. 2002, 35, 131–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klingenberg, C.P. Developmental Instability as a Research Tool: Using Patterns of Fluctuating Asymmetry to Infer the Developmental Origins of Morphological Integration. In Developmental Stability: Causes andConsequences; Klingeberg, C.P., Polak, M., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 427–442. ISBN 978-0195143454. [Google Scholar]
- Klingenberg, C.P.; Zaklan, S.D. Morphological Integration between Developmental Compartments in the Drosophila Wing. Evolution 2000, 54, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Breuker, C.J.; Patterson, J.S.; Klingenberg, C.P. A Single Basis for Developmental Buffering of Drosophila Wing Shape. PLoS ONE 2006, 1, e7. [Google Scholar] [CrossRef]
- Debat, V.; Alibert, P.; David, P.; Paradis, E.; Auffray, J.-C. Independence between Developmental Stability and Canalization in the Skull of the House Mouse. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Willmore, K.E.; Klingenberg, C.P.; Hallgrímsson, B. The Relationship between Fluctuating Asymmetry and Environmental Variance in Rhesus Macaque Skulls. Evolution 2005, 59, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, M.; Takahashi, K.H. Natural Genetic Variation in Fluctuating Asymmetry of Wing Shape in Drosophila melanogaster. Ecol. Res. 2012, 27, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Radwan, J.; Watson, P.J.; Farslow, J.; Thornhill, R. Procrustean Analysis of Fluctuating Asymmetry in the Bulb Mite Rhizoglyphus robini Claparede (Astigmata: Acaridae). Biol. J. Linn. Soc. 2003, 80, 499–505. [Google Scholar] [CrossRef]
- Leamy, L.J.; Klingenberg, C.P. The Genetics and Evolution of Fluctuating Asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Carter, A.J.R.; Houle, D. Artificial Selection Reveals Heritable Variation for Developmental Instability. Evolution 2011, 65, 3558–3564. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Thornhill, R. A Meta-Analysis of the Heritability of Developmental Stability. J. Evol. Biol. 1997, 10, 1–16. [Google Scholar] [CrossRef]
- Jeffery, N.; Spoor, F. Prenatal Growth and Development of the Modern Human Labyrinth. J. Anat. 2004, 204, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Ekdale, E.G. Ontogenetic Variation in the Bony Labyrinth of Monodelphis domestica (Mammalia: Marsupialia) Following Ossification of the Inner Ear Cavities. Anat. Rec. 2010, 293, 1896–1912. [Google Scholar] [CrossRef]
- Berlioz, E.; Cornette, R.; Lenoir, N.; Santin, M.D.; Lehmann, T. Exploring the Ontogenetic Development of the Inner Ear in Aardvarks. J. Anat. 2021, 238, 1128–1142. [Google Scholar] [CrossRef] [PubMed]
- Mennecart, B.; Costeur, L. Shape Variation and Ontogeny of the Ruminant Bony Labyrinth, an Example in Tragulidae. J. Anat. 2016, 229, 422–435. [Google Scholar] [CrossRef] [Green Version]
- Costeur, L.; Mennecart, B.; Müller, B.; Schulz, G. Prenatal Growth Stages Show the Development of the Ruminant Bony Labyrinth and Petrosal Bone. J. Anat. 2017, 230, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, S.; Shiraki, N.; Yamada, S.; Uwabe, C.; Imai, H.; Matsuda, T.; Yoneyama, A.; Takeda, T.; Takakuwa, T. Morphogenesis of the Inner Ear at Different Stages of Normal Human Development. Anat. Rec. 2015, 298, 2081–2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, H.-Y.; Chen, K.-G.; Yin, D.-M.; Hong, J.; Yang, L.; Zhang, T.-Y.; Dai, P.-D. The Age-Related Orientational Changes of Human Semicircular Canals. Clin. Exp. Otorhinolaryngol. 2016, 9, 109–115. [Google Scholar] [CrossRef] [PubMed]







| Measure | G. moho. | P. gran. | O. cras. | L. tard. | N. couc. | P. edwa. | |
|---|---|---|---|---|---|---|---|
| Left vestibulum | Mean(l_lat ∡ l_post) | 95.33 | 94.55 | 87.77 | 87.61 | 88.21 | 89.28 |
| VAR(l_lat ∡ l_post) | 4.30 | 11.67 | 7.64 | 36.17 | 22.04 | 30.78 | |
| Mean(l_lat ∡ l_ant) | 91.57 | 89.52 | 82.10 | 77.71 | 84.21 | 80.49 | |
| VAR(l_lat ∡ l_ant) | 5.05 | 7.13 | 11.68 | 25.84 | 26.41 | 40.55 | |
| Mean(l_ant ∡ l_post) | 94.35 | 94.63 | 97.80 | 92.85 | 91.44 | 95.44 | |
| VAR(l_ant ∡ l_post) | 4.06 | 4.31 | 10.94 | 10.57 | 12.72 | 11.09 | |
| Right vestibulum | Mean(r_lat ∡ r_post) | 95.40 | 94.17 | 87.61 | 87.35 | 87.63 | 89.65 |
| VAR(r_lat ∡ r_post) | 3.83 | 9.32 | 7.73 | 36.59 | 18.90 | 23.01 | |
| Mean(r_lat ∡ r_ant) | 91.05 | 89.27 | 82.04 | 77.46 | 85.43 | 80.74 | |
| VAR(r_lat ∡ r_ant) | 5.58 | 6.30 | 13.29 | 23.10 | 29.12 | 29.25 | |
| Mean(r_ant ∡ r_post) | 94.67 | 94.61 | 97.85 | 92.74 | 91.54 | 95.73 | |
| VAR(r_ant ∡ r_post) | 4.19 | 4.42 | 9.68 | 10.09 | 14.26 | 12.39 | |
| Both vestibuli | Mean(6 ipsilateral ∡) | 93.7 | 92.8 | 89.2 | 86.0 | 88.1 | 88.6 |
| Synergistic pairs of SCCs | Mean(l_lat ∡ r_lat) | 4.39 | 6.64 | 11.90 | 13.00 | 9.13 | 11.58 |
| VAR(l_lat ∡ r_lat) | 16.20 | 12.55 | 42.64 | 122.09 | 30.78 | 97.10 | |
| Mean(l_ant ∡ r_post) | 6.09 | 5.05 | 5.21 | 11.13 | 11.37 | 12.27 | |
| VAR(l_ant ∡ r_post) | 5.08 | 5.38 | 7.27 | 49.65 | 32.01 | 28.52 | |
| Mean(l_post ∡ r_ant) | 6.28 | 4.97 | 5.25 | 10.42 | 11.36 | 12.17 | |
| VAR(l_post ∡ r_ant) | 5.67 | 5.05 | 6.70 | 60.17 | 34.46 | 28.58 | |
| Asymmetry | Mean(Asym(lat ∡ post)) | 0.78 | 0.68 | 1.04 | 1.30 | 1.50 | 1.23 |
| VAR(Asym(lat ∡ post)) | 0.39 | 0.35 | 0.53 | 0.51 | 1.21 | 0.52 | |
| Mean(Asym(lat ∡ ant)) | 0.70 | 0.76 | 1.23 | 1.14 | 1.79 | 1.94 | |
| VAR(Asym(lat ∡ ant)) | 0.39 | 0.52 | 1.51 | 0.94 | 2.41 | 2.45 | |
| Mean(Asym(ant ∡ post)) | 0.79 | 0.63 | 0.81 | 0.73 | 1.06 | 1.06 | |
| VAR(Asym(ant ∡ post)) | 0.35 | 0.24 | 0.37 | 0.33 | 1.27 | 0.84 |
| Measure | G. moho. | P. gran. | O. cras. | L. tard. | N. couc. | P. edwa. | |
|---|---|---|---|---|---|---|---|
| Left vestibulum | Mean(CS(l_lat)) | 8.66 | 8.39 | 9.48 | 5.88 | 7.36 | 7.62 |
| VAR(RSC(CS(l_lat))) | 0.0019 | 0.0037 | 0.0021 | 0.0050 | 0.0063 | 0.0081 | |
| Mean(CS(l_ant)) | 8.24 | 7.88 | 10.88 | 7.83 | 10.10 | 10.06 | |
| VAR(RSC(CS(l_ant))) | 0.0012 | 0.0021 | 0.0016 | 0.0031 | 0.0054 | 0.0074 | |
| Mean(CS(l_post)) | 7.80 | 7.78 | 10.06 | 7.19 | 8.47 | 8.85 | |
| VAR(RSC(CS(l_post))) | 0.0022 | 0.0031 | 0.0016 | 0.0032 | 0.0055 | 0.0064 | |
| Mean(l_CCL) | 01.03 | 01.07 | 1.67 | 1.62 | 1.50 | 1.58 | |
| VAR(RSC(l_CCL)) | 0.0085 | 0.0084 | 0.0089 | 0.0086 | 0.0619 | 0.0540 | |
| Right vestibulum | Mean(CS(r_lat)) | 8.66 | 8.40 | 9.51 | 5.91 | 7.34 | 7.66 |
| VAR(RSC(CS(r_lat))) | 0.0019 | 0.0038 | 0.0023 | 0.0050 | 0.0060 | 0.0082 | |
| Mean(CS(r_ant)) | 8.27 | 7.86 | 10.84 | 7.85 | 10.10 | 10.03 | |
| VAR(RSC(CS(r_ant))) | 0.0013 | 0.0017 | 0.0016 | 0.0035 | 0.0054 | 0.0074 | |
| Mean(CS(r_post)) | 7.82 | 7.78 | 10.08 | 7.14 | 8.45 | 8.87 | |
| VAR(RSC(CS(r_post))) | 0.0021 | 0.0031 | 0.0018 | 0.0030 | 0.0050 | 0.0060 | |
| Mean(r_CCL) | 01.09 | 1.13 | 1.62 | 1.60 | 1.56 | 1.59 | |
| VAR(RSC(r_CCL)) | 0.0064 | 0.0076 | 0.0070 | 0.0108 | 0.0342 | 0.0572 | |
| Asymmetry | Mean(Asym(RSC(CS(lat)))) | 0.0047 | 0.0060 | 0.0109 | 0.0117 | 0.0069 | 0.0145 |
| VAR(Asym(RSC(CS(lat)))) | 0.000011 | 0.000017 | 0.000069 | 0.000076 | 0.000021 | 0.000086 | |
| Mean(Asym(RSC(CS(ant)))) | 0.0071 | 0.0068 | 0.0064 | 0.0116 | 0.0118 | 0.0098 | |
| VAR(Asym(RSC(CS(ant)))) | 0.000026 | 0.000018 | 0.000030 | 0.000108 | 0.000069 | 0.000069 | |
| Mean(Asym(RSC(CS(post)))) | 0.0071 | 0.0031 | 0.0096 | 0.0108 | 0.0117 | 0.0091 | |
| VAR(Asym(RSC(CS(post)))) | 0.000023 | 0.000006 | 0.000053 | 0.000049 | 0.000104 | 0.000067 | |
| Mean(Asym(RSC(CCL))) | 0.0709 | 0.0587 | 0.0446 | 0.0287 | 0.0895 | 0.0518 | |
| VAR(Asym(RSC(CCL))) | 0.0017 | 0.0013 | 0.0015 | 0.0008 | 0.0031 | 0.0019 |
| G. moholi | P. granti | O. crassicaudatus | L. tardigradus | N. coucang | P. edwardsi | |
|---|---|---|---|---|---|---|
| 3 SCCs SV | 0.0015 | 0.0018 | 0.0020 | 0.0044 | 0.0039 | 0.0054 |
| 3 SCCs FA10 | 0.00035 | 0.00026 | 0.00046 | 0.00062 | 0.00099 | 0.00083 |
| LSCC SV | 0.0009 | 0.0013 | 0.0013 | 0.0029 | 0.0022 | 0.0043 |
| LSCC FA10 | 0.00045 | 0.00037 | 0.00059 | 0.00132 | 0.00087 | 0.00106 |
| ASCC SV | 0.0011 | 0.001 | 0.0011 | 0.0028 | 0.0023 | 0.0037 |
| ASCC FA10 | 0.00030 | 0.00021 | 0.00037 | 0.00055 | 0.00107 | 0.00086 |
| PSCC SV | 0.0013 | 0.0012 | 0.001 | 0.003 | 0.0022 | 0.0033 |
| PSCC FA10 | 0.00043 | 0.00024 | 0.00038 | 0.00054 | 0.00100 | 0.00083 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebrun, R.; Perier, A.; Masters, J.; Marivaux, L.; Couette, S. Lower Levels of Vestibular Developmental Stability in Slow-Moving than Fast-Moving Primates. Symmetry 2021, 13, 2305. https://0-doi-org.brum.beds.ac.uk/10.3390/sym13122305
Lebrun R, Perier A, Masters J, Marivaux L, Couette S. Lower Levels of Vestibular Developmental Stability in Slow-Moving than Fast-Moving Primates. Symmetry. 2021; 13(12):2305. https://0-doi-org.brum.beds.ac.uk/10.3390/sym13122305
Chicago/Turabian StyleLebrun, Renaud, Alexandre Perier, Judith Masters, Laurent Marivaux, and Sébastien Couette. 2021. "Lower Levels of Vestibular Developmental Stability in Slow-Moving than Fast-Moving Primates" Symmetry 13, no. 12: 2305. https://0-doi-org.brum.beds.ac.uk/10.3390/sym13122305