In Vivo Estimation of the Biological Effects of Endocrine Disruptors in Rabbits after Combined and Long-Term Exposure: Study Protocol
Abstract
:1. Introduction
- Glyphosate
- Bisphenol A
- Parabens
- Triclosan
- Bis (2-ethylhexyl) phthalate
2. Study Design
2.1. Study Characteristics
2.2. Study Group
2.3. Sample Size Estimation
2.4. Breeding-Administration-Dosages
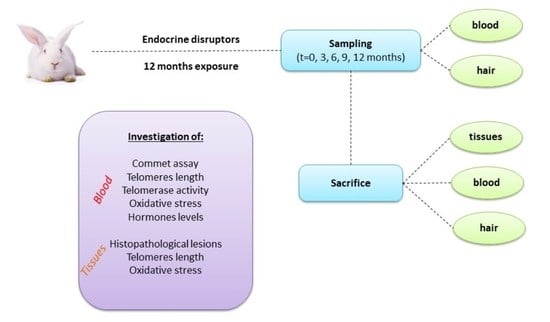
2.5. Sampling-Sacrifice
- Micronuclei assay
- Reproductive hormone levels
- Telomere length
- Telomerase activity
- Oxidative stress
- Comet assay
3. Discussion
4. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- European Food Safety Authority. Scientific Opinion on the hazard assessment of endocrine disruptors: Scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. EFSA J. 2013, 11, 3132. [Google Scholar]
- Tsatsakis, A.; Kouretas, D.; Tzatzarakis, M.; Stivaktakis, P.; Tsarouhas, K.; Golokhvast, K.; Rakitskii, V.; Tutelyan, V.; Hernandez, A.; Rezaee, R.; et al. Simulating real-life exposures to uncover possible risks to human health: A proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2016, 36, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.F.; Tsatsakis, A.M. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [Green Version]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of the herbicide glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Blumberg, B.; Antoniou, M.N.; Benbrook, C.M.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; et al. Is it time to reassess current safety standards for glyphosate-based herbicides? J. Epidemiol. Community Health 2017, 71, 613–618. [Google Scholar] [CrossRef]
- Michałowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Tzatzarakis, M.N.; Karzi, V.; Vakonaki, E.; Goumenou, M.; Kavvalakis, M.; Stivaktakis, P.; Tsitsimpikou, C.; Tsakiris, I.; Rizos, A.K.; Tsatsakis, A.M. Bisphenol A in soft drinks and canned foods and data evaluation. Food Addit. Contam. Part B 2016, 10, 85–90. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Dodge, L.E.; Kelley, K.E.; Williams, P.L.; Williams, M.A.; Hernández-Díaz, S.; Missmer, S.A.; Hauser, R. Medications as a source of paraben exposure. Reprod. Toxicol. 2015, 52, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, K.; Ratajczak–Wrona, W.; Górska, M.; Jabłońska, E. Parabens and their effects on the endocrine system. Mol. Cell. Endocrinol. 2018, 474, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Weatherly, L.M.; Gosse, J.A. Triclosan exposure, transformation, and human health effects. J. Toxicol. Environ. Health Part B 2017, 20, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Bedoux, G.; Roig, B.; Thomas, O.; Dupont, V.; Le Bot, B. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment. Environ. Sci. Pollut. Res. 2011, 19, 1044–1065. [Google Scholar] [CrossRef] [PubMed]
- Rowdhwal, S.S.S.; Chen, J. Toxic Effects of Di-2-ethylhexyl Phthalate: An Overview. BioMed. Res. Int. 2018, 2018, 1750368. [Google Scholar] [CrossRef] [Green Version]
- Erythropel, H.C.; Maric, M.; Nicell, J.A.; Leask, R.L.; Yargeau, V. Leaching of the plasticizer di(2-ethylhexyl) phthalate (DEHP) from plastic containers and the question of human exposure. Appl. Microbiol. Biotechnol. 2014, 98, 9967–9981. [Google Scholar] [CrossRef]
- Romano, R.M.; Romano, M.A.; Bernardi, M.M.; Furtado, P.V.; Oliveira, C.A. Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology. Reprod. Toxicol. 2010, 84, 309–317. [Google Scholar] [CrossRef]
- Owagboriayea, F.O.; Dedeke, G.A.; Ademolu, K.O.; Olujimi, O.O.; Ashidi, J.S.; Adeyinka, A.A. Reproductive toxicity of Roundup herbicide exposure in male albino rat. Exper. Toxicol. Pathol. 2017, 69, 461–468. [Google Scholar] [CrossRef]
- Ghisi, N.D.C.; De Oliveira, E.C.; Prioli, A.J. Does exposure to glyphosate lead to an increase in the micronuclei frequency? A systematic and meta-analytic review. Chemosphere 2016, 145, 42–54. [Google Scholar] [CrossRef]
- Louzon, M.; Coeurdassier, M.; Gimbert, F.; Pauget, B.; de Vaufleury, A. Telomere dynamic in humans and animals: Review and perspectives in environmental toxicology. Environ. Int. 2019, 131, 105025. [Google Scholar] [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984, 105, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Keles, M.; Taysi, S.; Sen, N.; Aksoy, H.; Akçay, F. Effect of Corticosteroid Therapy on Serum and CSF Malondialdehyde and Antioxidant Proteins in Multiple Sclerosis. Can. J. Neurol. Sci. 2001, 28, 141–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Patsoukis, N.; Zervoudakis, G.; Panagopoulos, N.T.; Georgiou, C.D.; Angelatou, F.; A Matsokis, N. Thiol redox state (TRS) and oxidative stress in the mouse hippocampus after pentylenetetrazol-induced epileptic seizure. Neurosci. Lett. 2004, 357, 83–86. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood. Biomarkers 2016, 21, 208–217. [Google Scholar] [CrossRef]
- Ledda, C.; Cannizzaro, E.; Cinà, D.; Filetti, V.; Vitale, E.; Paravizzini, G.; Di Naso, C.; Iavicoli, I.; Rapisarda, V. Oxidative stress and DNA damage in agricultural workers after exposure to pesticides. J. Occup. Med. Toxicol. 2021, 16, 1. [Google Scholar] [CrossRef]
- Puttabyatappa, M.; Banker, M.; Zeng, L.; Goodrich, J.M.; E Domino, S.; Dolinoy, D.C.; Meeker, J.D.; Pennathur, S.; Song, P.X.K.; Padmanabhan, V. Maternal Exposure to Environmental Disruptors and Sexually Dimorphic Changes in Maternal and Neonatal Oxidative Stress. J. Clin. Endocrinol. Metab. 2019, 105, 492–505. [Google Scholar] [CrossRef]
- Kara, M.; Oztas, E.; Ramazanoğulları, R.; Kouretas, D.; Nepka, C.; Tsatsakis, A.; Veskoukis, A.S. Benomyl, a benzimidazole fungicide, induces oxidative stress and apoptosis in neural cells. Toxicol. Rep. 2020, 7, 501–509. [Google Scholar] [CrossRef]
- Machtinger, R.; Berman, T.; Adir, M.; Mansur, A.; Baccarelli, A.A.; Racowsky, C.; Calafat, A.M.; Hauser, R.; Nahum, R. Urinary concentrations of phthalate metabolites, bisphenols and personal care product chemical biomarkers in pregnant women in Israel. Environ. Int. 2018, 116, 319–325. [Google Scholar] [CrossRef]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef]
- Parvez, S.; Gerona, R.R.; Proctor, C.; Friesen, M.; Ashby, J.L.; Reiter, J.L.; Lui, Z.; Winchester, P.D. Glyphosate exposure in pregnancy and shortened gestational length: A prospective Indiana birth cohort study. Environ. Health 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Błędzka, D.; Gromadzińska, J.; Wąsowicz, W. Parabens. From environmental studies to human health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Katsikantami, I.; Sifakis, S.; Tzatzarakis, M.N.; Vakonaki, E.; Kalantzi, O.I.; Tsatsakis, A.M.; Rizos, A.K. A global assessment of phthalates burden and related links to health effects. Environ. Int. 2016, 97, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Rodricks, J.V.; Swenberg, J.A.; Borzelleca, J.F.; Maronpot, R.R.; Shipp, A.M. Triclosan: A critical review of the experimental data and development of margins of safety for consumer products. Crit. Rev. Toxicol. 2010, 40, 422–484. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Docea, A.O.; Constantin, C.; Calina, D.; Zlatian, O.; Nikolouzakis, T.K.; Stivaktakis, P.D.; Kalogeraki, A.; Liesivuori, J.; Tzanakakis, G.; et al. Genotoxic, cytotoxic, and cytopathological effects in rats exposed for 18 months to a mixture of 13 chemicals in doses below NOAEL levels. Toxicol. Lett. 2019, 316, 154–170. [Google Scholar] [CrossRef]
- Fountoucidou, P.; Veskoukis, A.S.; Kerasioti, E.; Docea, A.O.; Taitzoglou, I.A.; Liesivuori, J.; Tsatsakis, A.; Kouretas, D. A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: The time and dose issue. Toxicol. Lett. 2019, 317, 24–44. [Google Scholar] [CrossRef]
- Tsiaoussis, J.; Antoniou, M.N.; Koliarakis, I.; Mesnage, R.; Vardavas, C.I.; Izotov, B.N.; Psaroulaki, A.; Tsatsakis, A. Effects of single and combined toxic exposures on the gut microbiome: Current knowledge and future directions. Toxicol. Lett. 2019, 312, 72–97. [Google Scholar] [CrossRef]
- Gress, S.; Lemoine, S.; Séralini, G.E.; Puddu, P.E. Glyphosate-based herbicides potently affect cardiovascular system in mammals: Review of the literature. Cardiovasc. Toxicol. 2015, 15, 117–126. [Google Scholar] [CrossRef]
- Defarge, N.; de Vendômois, J.S.; Séralini, G.E. Toxicity of formulants and heavy metals in glyphosate-based herbicides and other pesticides. Tox. Rep. 2018, 5, 156–163. [Google Scholar] [CrossRef]
- Bach, N.C.; Marino, D.J.G.; Natale, G.S.; Somoza, G.M. Effects of Glyphosate and its commercial formulation, Roundup® Ultramax, on liver histology of tadpoles of the Neotropical frog, Leptodactylus latrans (Amphibia: Anura). Chemosphere 2018, 202, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karzi, V.; Tzatzarakis, M.N.; Alegakis, A.; Vakonaki, E.; Fragkiadoulaki, I.; Kaloudis, K.; Chalkiadaki, C.; Apalaki, P.; Panagiotopoulou, M.; Kalliantasi, A.; et al. In Vivo Estimation of the Biological Effects of Endocrine Disruptors in Rabbits after Combined and Long-Term Exposure: Study Protocol. Toxics 2022, 10, 246. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10050246
Karzi V, Tzatzarakis MN, Alegakis A, Vakonaki E, Fragkiadoulaki I, Kaloudis K, Chalkiadaki C, Apalaki P, Panagiotopoulou M, Kalliantasi A, et al. In Vivo Estimation of the Biological Effects of Endocrine Disruptors in Rabbits after Combined and Long-Term Exposure: Study Protocol. Toxics. 2022; 10(5):246. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10050246
Chicago/Turabian StyleKarzi, Vasiliki, Manolis N. Tzatzarakis, Athanasios Alegakis, Elena Vakonaki, Irene Fragkiadoulaki, Konstantinos Kaloudis, Christina Chalkiadaki, Paraskevi Apalaki, Maria Panagiotopoulou, Aikaterini Kalliantasi, and et al. 2022. "In Vivo Estimation of the Biological Effects of Endocrine Disruptors in Rabbits after Combined and Long-Term Exposure: Study Protocol" Toxics 10, no. 5: 246. https://0-doi-org.brum.beds.ac.uk/10.3390/toxics10050246