Venom in Furs: Facial Masks as Aposematic Signals in a Venomous Mammal
Abstract
:1. Introduction
2. Results
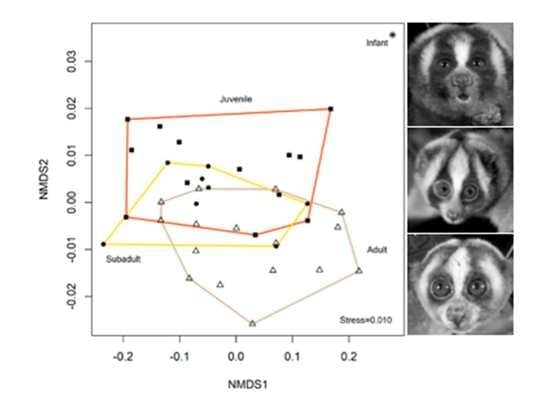
2.1. Colour
2.2. Aggressiveness
3. Discussion
3.1. Cryptic Anti-Predator Function
3.2. Intraspecific Competition
3.3. Colour Advertising Young Animals
4. Conclusions
5. Materials and Methods
Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ortolani, A. Spots, stripes, tail tips and dark eyes: Predicting the function of carnivore colour patterns using the comparative method. Biol. J. Linn. Soc. Lond. 1999, 67, 433–476. [Google Scholar] [CrossRef]
- Ancillotto, L.; Mori, E. Adaptive significance of coat colouration and patterns of Sciuromorpha (Rodentia). Ethol. Ecol. Evol. 2016, 29, 1–14. [Google Scholar] [CrossRef]
- Caro, T. Contrasting coloration in terrestrial mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Stankowich, T.; Caro, T.; Cox, M. Bold coloration and the evolution of aposematism in terrestrial carnivores. Evolution 2011, 65, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.; Halpin, C. Why are warning displays multimodal? Behav. Ecol. Sociobiol. 2013, 67, 1425–1439. [Google Scholar] [CrossRef]
- Stevens, M. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Sci. B Biol. 2007, 274, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Mappes, J.; Marples, N.; Endler, J.A. The complex business of survival by aposematism. Trends Ecol. Evol. 2005, 20, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Gohli, J.; Högstedt, G. Explaining the evolution of warning coloration: Secreted secondary defence chemicals may facilitate the evolution of visual aposematic signals. PLoS ONE 2009, 4, e5779. [Google Scholar] [CrossRef] [PubMed]
- Caro, T. The colour of extant mammals. Semin. Cell Dev. Biol. 2013, 24, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Caro, T.; Walker, H.; Rossman, Z.; Hendrix, M.; Stankowich, T. Why is the giant panda black and white? Behav. Ecol. 2017, 28, 657–667. [Google Scholar] [CrossRef]
- Newman, C.; Buesching, C.D.; Wolff, J.O. The function of facial masks in “midguild” carnivores. Oikos 2005, 108, 623–633. [Google Scholar] [CrossRef]
- Süsstrunk, S.; Buckley, R.; Swen, S. Standard RGB color spaces. Soc. Imaging Sci. Technol. 1999, 1, 127–134. [Google Scholar]
- Jacobs, G.H. Primate photopigments and primate color vision. Proc. Natl. Acad. Sci. USA 1996, 93, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Chatty, S.; Sire, S.; Vinot, J.L.; Lecoanet, P.; Lemort, A.; Mertz, C. Revisiting visual interface programming: Creating GUI tools for designers and programmers. In Proceedings of the 17th Annual ACM Symposium on User Interface Software and Technology, Santa Fe, NM, USA, 24–27 October 2004; ACM: New York, NY, USA, 2004. [Google Scholar]
- Cheng, M.M.; Mitra, N.J.; Huang, X.; Torr, P.H.; Hu, S.M. Global contrast based salient region detection. IEEE Trans. Pattern Anal. Mach. Intell. 2016, 37, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Gamberale-Stille, G.; Guilford, T. Contrast versus colour in aposematic signals. Anim. Behav. 2003, 65, 1021–1026. [Google Scholar] [CrossRef]
- Maan, M.E.; Cummings, M.E. Female preferences for aposematic signal components in a polymorphic poison frog. Evolution 2008, 62, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Crothers, L.; Gering, E.; Cummings, M. Aposematic signal variation predicts male–male interactions in a polymorphic poison frog. Evolution 2011, 65, 599–605. [Google Scholar] [CrossRef]
- Brandley, N.; Johnson, M.; Johnsen, S. Aposematic signals in North American black widows are more conspicuous to predators than to prey. Behav. Ecol. 2016, 27, 1104–1112. [Google Scholar] [CrossRef]
- Neal, T.J. A test of the function of juvenile color patterns in the Pomacentrid Fish Hypsypops rubicundus (Teleostei: Pomacentridae). Pac. Sci. 1993, 47, 240–247. [Google Scholar]
- Nekaris, K.A.I. Extreme primates: Ecology and evolution of Asian lorises. Evol. Anthropol. 2014, 23, 177–187. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Moore, R.S.; Rode, E.J.; Fry, B.G. Mad, bad and dangerous to know: The biochemistry, ecology and evolution of slow loris venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Poindexter, S.A.; Reinhardt, K.D.; Nijman, V.; Nekaris, K.A.I. Slow lorises (Nycticebus spp.) display evidence of handedness in the wild and in captivity. Laterality 2018, 6, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Alterman, L. Toxins and Toothcombs: Potential Allospecific Chemical Defenses in Nycticebus and Perodicticus. In Creatures of the Dark; Alterman, L., Doyle, G.A., Izard, M.K., Eds.; Springer: Boston, MA, USA, 1995; pp. 413–424. Available online: https://0-link-springer-com.brum.beds.ac.uk/chapter/10.1007/978-1-4757-2405-9_24 (accessed on 18 December 2018).
- Nekaris, K.A.I.; Pimley, E.R.; Ablard, K.M. Predator defense by slender lorises and pottos. In Primate Anti-Predator Strategies; Gursky-Doyen, S., Nekaris, K.A.I., Eds.; Springer: Boston, MA, USA, 2007; pp. 222–240. Available online: https://0-www-springer-com.brum.beds.ac.uk/gb/book/9780387348070 (accessed on 18 December 2018).
- Nekaris, K.A.I.; Jaffe, S. Unexpected diversity of slow lorises (Nycticebus spp.) within the Javan pet trade implications for slow loris taxonomy. Contrib. Zool. 2007, 76, 187–196. [Google Scholar]
- Fuller, G.; Eggen, W.F.; Wirdateti, W.; Nekaris, K.A.I. Welfare impacts of the illegal wildlife trade in a cohort of confiscated greater slow lorises, Nycticebus coucang. J. Appl. Anim. Welf. Sci. 2018, 21, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Madani, G.; Nekaris, K.A.I. Anaphylactic shock following the bite of a wild Kayan slow loris (Nycticebus kayan): Implications for slow loris conservation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Cordero, J.M.; Moreno-Rueda, G.; López-Orta, A.; Marfil-Daza, C.; Ros-Santaella, J.L.; Ortiz-Sánchez, F.J. Brighter-colored paper wasps (Polistes dominula) have larger poison glands. Front. Zool. 2012, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Creer, D.A. Correlations between ontogenetic change in color pattern and antipredator behavior in the racer, Coluber constrictor. Ethology 2005, 111, 287–300. [Google Scholar] [CrossRef]
- Speed, M.P.; Ruxton, G.D. How bright and how nasty: Explaining diversity in warning signal strength. Evolution 2007, 61, 623–635. [Google Scholar] [CrossRef]
- Geerah, D.R.; O’Hagan, R.P.; Wirdateti, W.; Nekaris, K.A.I. The use of ultrasonic communication to maintain social cohesion in the Javan slow loris (Nycticebus javanicus). Folia Primatol. 2019, in press. [Google Scholar]
- Wüster, W.; Duarte, M.R.; da Graça Salomao, M. Morphological correlates of incipient arboreality and ornithophagy in island pit vipers, and the phylogenetic position of Bothrops insularis. J. Zool. 2005, 266, 1–10. [Google Scholar] [CrossRef]
- Endler, J.A.; Mappes, J. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 2004, 163, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Thompson, V. Polymorphism under apostatic and aposematic selection. Heredity 1984, 53, 677. [Google Scholar] [CrossRef]
- Endler, J.A. The place of hybridization in evolution. Evolution 1998, 52, 640–644. [Google Scholar] [CrossRef]
- Marshall, K.L.; Gluckman, T.L. The evolution of pattern camouflage strategies in waterfowl and game birds. Ecol. Evol. 2015, 5, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Mäthger, L.M.; Bell, G.R.; Kuzirian, A.M.; Allen, J.J.; Hanlon, R.T. How does the blue-ringed octopus (Hapalochlaena lunulata) flash its blue rings? J. Exp. Biol. 2012, 215, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, M.; Weldon, A.; Poindexter, S.A.; Gibson, N.; Nekaris, K.A.I. Survey of practitioners handling slow lorises (Primates: Nycticebus): An assessment of the harmful effects of slow loris bites. J. Venom. Res. 2018, 9, 1–7. [Google Scholar]
- Forsman, A. Opposing fitness consequences of colour pattern in male and female snakes. J. Evol. Biol. 1995, 8, 53–70. [Google Scholar] [CrossRef]
- Újvári, B.; Lazányi, I.; Farkas, B.; Korsós, Z. An Isolated Adder (Vipera berus) Population in Hungary; Herpetologia Candiana; Natural History Museum of Crete & SHE: Irakleio, Greece, 2001; pp. 127–135. [Google Scholar]
- Caro, T.; Stankowich, T.; Mesnick, S.L.; Costa, D.P.; Beeman, K. Pelage coloration in pinnipeds: Functional considerations. Behav. Ecol. 2012, 23, 765–774. [Google Scholar] [CrossRef]
- Andersson, M. Aposematism and crypsis in a rodent: Antipredator defence of the Norwegian lemming. Behav. Ecol. Sociobiol. 2015, 69, 571–581. [Google Scholar] [CrossRef]
- Taitt, M.J. Adaptive coloration in Lemmus lemmus: Why aren’t Norwegian lemmings brown. In The Biology of Lemmings; Stenseth, N.C., Ims, R.A., Eds.; Academic Press: London, UK, 1993; pp. 439–445. [Google Scholar]
- Mackessy, S.P. Venom ontogeny in the Pacific rattlesnakes Crotalus viridis helleri and C. v. oreganus. Copeia 1988, 1, 92–101. Available online: https://0-www-jstor-org.brum.beds.ac.uk/stable/1445927?seq=1#metadata_info_tab_contents (accessed on 18 December 2018). [CrossRef]
- Kelley, L.A.; Kelley, J.L. Perceptual biases and animal illusions: A response to comments on Kelley and Kelley. Behav. Ecol. 2014, 25, 468–469. [Google Scholar] [CrossRef]
- Bearder, S.K. Physical and social diversity among nocturnal primates: A new view based on long term research. Primates 1999, 40, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Hart, D. Predation on primates: A biogeographical analysis. In Primate Anti-Predator Strategies; Gursky, S.L., Nekaris, K.A.I., Eds.; Springer: Boston, MA, USA, 2007; pp. 27–59. [Google Scholar]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Rode-Margono, E.J.; Nekaris, K.A.I. Impact of climate and moonlight on a venomous mammal, the Javan slow loris (Nycticebus javanicus Geoffroy, 1812). Contrib. Zool. 2014, 83, 217–225. [Google Scholar]
- Poindexter, S.A.; Nekaris, K.A.I. Vertical clingers and gougers: Rapid acquisition of adult limb proportions facilitates feeding behaviours in young Javan slow lorises (Nycticebus javanicus). Mamm. Biol. 2017, 87, 40–49. [Google Scholar] [CrossRef]
- Munds, R.A.; Nekaris, K.A.I.; Ford, S.M. Taxonomy of the Bornean slow loris, with new species Nycticebus kayan (Primates, Lorisidae). Am. J. Primatol. 2013, 75, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Samia, D.S.; Francini, R.B. An affordable method to measure animal-background contrast using digital images. Int. J. Fauna Biol. Stud. 2015, 2, 8–16. [Google Scholar]
- Stevens, M.; Rong, C.P.; Todd, P.A. Colour change and camouflage in the horned ghost crab Ocypode ceratophthalmus. Biol. J. Linn. Soc. Lond. 2013, 109, 257–270. [Google Scholar] [CrossRef]
- Ruck, L.; Brown, C.T. Quantitative analysis of Munsell color data from archeological ceramics. J. Archaeol. Sci. Rep. 2015, 3, 549–557. [Google Scholar] [CrossRef]
- Stasinopoulos, D.M.; Rigby, R.A. Generalized additive models for location scale and shape (GAMLSS) in R. J. Stat. Softw. 2007, 23, 1–46. [Google Scholar] [CrossRef]





| Model | Estimate | Std. Error | t Value | p Value |
|---|---|---|---|---|
| Aggressiveness level ~ | ||||
| Sex | 0.188 | 0.071 | 2.66 | 0.008 * |
| Age | −0.126 | 0.0005 | 2.19 | 0.030 * |
| Presence of wounds | −0.168 | 0.079 | −2.12 | 0.035 * |
| Brachial gland secretion | −0.003 | 0.064 | −0.06 | 0.951 |
| Number of captures | −0.025 | 0.008 | −2.87 | 0.004 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekaris, K.A.-I.; Weldon, A.; Imron, M.A.; Maynard, K.Q.; Nijman, V.; Poindexter, S.A.; Morcatty, T.Q. Venom in Furs: Facial Masks as Aposematic Signals in a Venomous Mammal. Toxins 2019, 11, 93. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11020093
Nekaris KA-I, Weldon A, Imron MA, Maynard KQ, Nijman V, Poindexter SA, Morcatty TQ. Venom in Furs: Facial Masks as Aposematic Signals in a Venomous Mammal. Toxins. 2019; 11(2):93. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11020093
Chicago/Turabian StyleNekaris, K. Anne-Isola, Ariana Weldon, Muhammad Ali Imron, Keely Q. Maynard, Vincent Nijman, Stephanie A. Poindexter, and Thais Queiroz Morcatty. 2019. "Venom in Furs: Facial Masks as Aposematic Signals in a Venomous Mammal" Toxins 11, no. 2: 93. https://0-doi-org.brum.beds.ac.uk/10.3390/toxins11020093