A Systematic Review on Viruses in Mass-Reared Edible Insect Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection Process (Information Sources, Search Strategy, Eligibility Criteria)
2.2. Summary of Extracted Data
3. Results
3.1. Coleoptera
3.1.1. Alphitobius diaperinus
3.1.2. Tenebrio molitor
3.1.3. Zophobas morio
3.2. Orthoptera
3.2.1. Acheta domesticus
3.2.2. Gryllodes sigillatus
3.2.3. Gryllus assimilis
3.2.4. Gryllus bimaculatus
3.2.5. Locusta migratoria
3.2.6. Schistocerca gregaria
3.2.7. Schistocerca americana
3.3. Diptera
3.3.1. Hermetia illucens
3.3.2. Musca domestica
3.4. Lepidoptera
3.4.1. Achroia grisella
3.4.2. Galleria mellonella
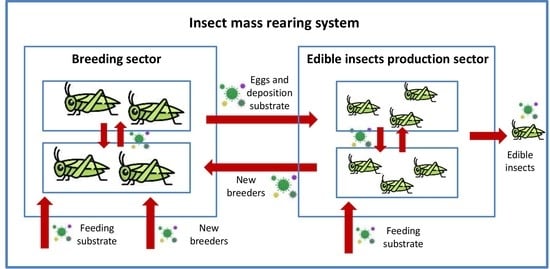
4. Prevention, Control and Management of Viruses in Edible Insect Mass-Rearing Facilities
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for food and feed security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; N. 171; pp. 1–201. Available online: https://www.fao.org/3/i3253e/i3253e.pdf (accessed on 29 October 2021).
- Van Huis, A. Potential of insects as food and feed in assuring food security. Ann. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Cadinu, L.A.; Barra, P.; Torre, F.; Delogu, F.; Madau, F.A. Insect rearing: Potential, challenges, and circularity. Sustainability 2020, 12, 4567. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insect Species of the World; Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands, 2017; Available online: http://www.entwurnl/UK/edible+insects/worldwide+species+list/ (accessed on 29 October 2021).
- Sun-Waterhouse, D.; Waterhouse, G.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- EFSA Scientific Committee. Scientific Opinion on a risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef] [Green Version]
- Murefu, T.R.; Macheka, L.; Musundire, R.; Manditsera, F.A. Safety of wild harvested and reared edible insects: A review. Food Control 2019, 101, 209–224. [Google Scholar] [CrossRef]
- Eilenberg, J.; van Oers, M.M.; Jensen, A.B.; Lecocq, A.; Maciel-Vergara, G.; Santacoloma, L.P.A.; van Loon, J.J.A.; Hesketh, H. Towards a coordination of European activities to diagnose and manage insect diseases in production facilities. J. Insects Food Feed 2018, 4, 157–166. [Google Scholar] [CrossRef]
- Wade, M.; Hoelle, J. A review of edible insect industrialization: Scales of production and implications for sustainability. Environ. Res. Lett. 2020, 15, 123013. [Google Scholar] [CrossRef]
- Wales, A.D.; Carrique-Mas, J.J.; Rankin, M.; Bell, B.; Thind, B.B.; Davies, R.H. Review of the carriage of zoonotic bacteria by arthropods, with special reference to Salmonella in mites, flies and litter beetles. Zoonoses Public Health 2010, 57, 299–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible Insects in a food safety and nutritional perspective: A critical review. Com. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Eilenberg, J.; Vlak, J.M.; Nielsen-LeRoux, C.; Cappellozza, S.; Jensen, A.B. Diseases in insects produced for food and feed. J. Insects Food Feed 2015, 1, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Camenzuli, L.; Belluco, S.; Meijer, N.; Ricci, A. Food Safety Issues Related to Uses of Insects for Feeds and Foods. Com. Rev. Food Sci. Food Saf. 2018, 17, 1172–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Com. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Dibusz, K.; Vejvodova, P. Systematic literature search to assist EFSA in the preparatory work for the safety assessment of Novel Food applications and Traditional Food notifications. EFSA Supp. Publ. 2020, 17, 1774E. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. OJL 2015, 327, 1–22. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:JOL_2015_327_R_0001 (accessed on 28 October 2021).
- European Union. European Union Commission implementing regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 64–71. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R2469 (accessed on 29 October 2021).
- European Union. Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/EC and Council Decision 92/438/EEC (Official Controls Regulation). Off. J. Eur. Union 2017, L95, 1–142. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32017R0625 (accessed on 28 October 2021).
- European Union. Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC. Off. J. Eur. Union 2019, L231, 1–28. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32019R1381 (accessed on 28 October 2021).
- European Union. Regulation (EC) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by-products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. Off. J. Eur. Union 2011, L54, 1–254. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2011.054.01.0001.01.ENG (accessed on 28 October 2021).
- Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Off. J. Eur. Union 2017, L 138, 92–115. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32017R0893 (accessed on 29 October 2021).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens; Turck, D.; Castenmiller, J.; De Henauw, S.; Ildico Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Vega, F.E. Scope and basic principles of insect pathology. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–12. [Google Scholar]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, E.; Santos, D.; Mingels, L.; Verdonckt, T.W.; Broeck, J.V. RNA Interference in Insects: Protecting Beneficials and Controlling Pests. Front. Physiol. 2019, 11, 1912. [Google Scholar] [CrossRef] [Green Version]
- The Insect Viruses; Miller, L.K.; Ball, L.A. (Eds.) Kluwer Academic Publishers Group: Dordrecht, The Netherlands, 1998. [Google Scholar] [CrossRef]
- Roossinck, M.J. The good viruses: Viral mutualistic symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef]
- Williams, T.; Bergoin, M.; van Oers, M.M. Diversity of large DNA viruses of invertebrates. J. Invertebr. Pathol. 2017, 147, 4–22. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Vaga, M.; Jansson, A.; Boqvist, S.; Vagsholm, I. The house cricket (Acheta domesticus) as a novel food: A risk profile. J. Insects Food Feed 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Wu, H.; Pang, R.; Cheng, T.; Xue, L.; Zeng, H.; Lei, T.; Chen, M.; Wu, S.; Ding, Y.; Zhang, J.; et al. Abundant and Diverse RNA Viruses in Insects Revealed by RNA-Seq Analysis: Ecological and Evolutionary Implications. Msystems 2020, 5, e00039-20. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.-H.; Chen, X.; Liu, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Rosario, K.; Mettel, K.A.; Benner, B.E.; Johnson, R.; Scott, C.; Yusseff-Vanegas, S.Z.; Baker, C.C.M.; Cassill, D.L.; Storer, C.; Varsani, A.; et al. Virus discovery in all three major lineages of terrestrial arthropods highlights the diversity of single-stranded DNA viruses associated with invertebrates. PeerJ 2018, 6, e5761. [Google Scholar] [CrossRef]
- Lü, P.; Pan, Y.; Yang, Y.; Zhu, F.; Li, C.; Guo, Z.; Yao, Q.; Chen, K. Discovery of anti-viral molecules and their vital functions in Bombyx mori. J. Invertebr. Pathol. 2018, 154, 12–18. [Google Scholar] [CrossRef]
- Martinet, J.-P.; Ferté, H.; Failloux, A.-B.; Schaffner, F.; Depaquit, J. Mosquitoes of North-Western Europe as Potential Vectors of Arboviruses: A Review. Viruses 2019, 11, 1059. [Google Scholar] [CrossRef] [Green Version]
- Öhlund, P.; Lundén, H.; Blomström, A.-L. Insect-specific virus evolution and potential effects on vector competence. Virus Genes 2019, 55, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Maciel-Vergara, G.; Ros, V.I.D. Viruses of insects reared for food and feed. J. Invertebr. Pathol. 2017, 147, 60–75. [Google Scholar] [CrossRef]
- Bonning, B.C. The Insect Virome: Opportunities and Challenges. Curr. Issues Mol. Biol. 2020, 34, 1–12. [Google Scholar] [CrossRef]
- Weissman, D.B.; Gray, D.A.; Pham, H.T.; Tijssen, P. Billions and billions sold: Pet-feeder crickets (Orthoptera: Gryllidae), commercial cricket farms, an epizootic densovirus, and government regulations make for a potential disaster. Zootaxa 2012, 3504, 67–88. [Google Scholar] [CrossRef]
- Weinmann, N.; Papp, T.; de Matos, A.P.A.; Teifke, J.P.; Marschang, R.E. Experimental Infection of Crickets (Gryllus Bimaculatus) with an Invertebrate Iridovirus Isolated from a High-Casqued Chameleon (Chamaeleo Hoehnelii). J. Vet. Diagn. Investig. 2007, 19, 674–679. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.A.; Skovgård, H.; Stockmarr, A.; Handberg, K.J.; Jørgensen, P.H. Persistence of Low-Pathogenic Avian Influenza H5N7 and H7N1 Subtypes in House Flies (Diptera: Muscidae). J. Med. Entomol. 2011, 48, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-de-Lima, V.H.; Lima-Camara, T.N. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: A systematic review. Parasit. Vectors 2018, 11, 77. [Google Scholar] [CrossRef]
- Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides Biting Midges-Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Bousquet, Y.; Thomas, D.B.; Bouchard, P.; Smith, A.D.; Aalbu, R.L.; Johnston, M.A.; Steiner, W.E., Jr. Catalogue of Tenebrionidae (Coleoptera) of North America. ZooKeys 2018, 728, 1–455. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Athanassiou, C.G. The Superworm, Zophobas morio (Coleoptera: Tenebrionidae): A ‘Sleeping Giant’ in Nutrient Sources. J. Insect Sci. 2021, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Mlcek, J.; Rop, O.; Borkovcova, M.; Bednarova, M.A. Comprehensive Look at the Possibilities of Edible Insects as Food in Europe—A Review. Pol. J. Food Nutr. Sci. 2014, 64, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Finke, M.D. Nutrient Composition of Bee Brood and its Potential as Human Food. Ecol. Food Nutr. 2005, 44, 257–270. [Google Scholar] [CrossRef]
- Tomotake, H.; Katagiri, M.; Yamato, M. Silkworm pupae (Bombyx mori) are new sources of high quality protein and lipid. J. Nutr. Sci. Vitaminol. 2010, 56, 446–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruun Jensen, A.; Evans, J.; Jonas-Levi, A.; Benjamin, O.; Martinez, I.; Dahle, B.; Roos, N.; Lecocq, A.; Foley, K. Standard methods for Apis mellifera brood as human food. J. Apicult. Res. 2019, 58, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.; Barbosa-Solomieu, V.; Chinchar, V.G. A Decade of Advances in Iridovirus Research. Adv. Virus Res. 2005, 65, 173–248. [Google Scholar] [CrossRef]
- Just, F.T.; Essbauer, S.S. Characterization of an Iridescent Virus Isolated from Gryllus bimaculatus (Orthoptera: Gryllidae). J. Invertebr. Pathol. 2001, 77, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Jakob, N.J.; Kleespies, R.G.; Tidona, C.A.; Müller, K.; Gelderblom, H.R.; Darai, G. Comparative analysis of the genome and host range characteristics of two insect iridoviruses: Chilo iridescent virus and a cricket iridovirus isolate. J. Gen. Virol. 2002, 83, 463–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, T. Natural invertebrate hosts of iridoviruses (Iridoviridae). Neotrop. Entomol. 2008, 37, 615–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp, T.; Marschang, R.E. Detection and Characterization of Invertebrate Iridoviruses Found in Reptiles and Prey Insects in Europe over the Past Two Decades. Viruses 2019, 11, 600. [Google Scholar] [CrossRef] [Green Version]
- Dunford, J.C.; Kaufman, P.E. Lesser Mealworm, Litter Beetle, Alphitobius diaperinus (Panzer) (Insecta: Coleoptera: Tenebrionidae); IFAS Extension, University of Florida: Gainesville, FL, USA, 2006; Available online: https://edis.ifas.ufl.edu/pdf/IN/IN662/IN662-D7vxrv2lkq.pdf (accessed on 28 October 2021).
- Esquivel, J.F.; Crippen, T.L.; Ward, L.A. Improved Visualization of Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae)—Part I: Morphological Features for Sex Determination of Multiple Stadia. Psyche J. Entomol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Dinev, I. The darkling beetle (Alphibotbius diaperinus)—A health hazard for broiler chicken production. Trakia J. Sci. 2013, 1, 1–4. Available online: http://www.uni-sz.bg/tsj/vol11N1_2013/Iv.Dinev.pdf (accessed on 5 November 2021).
- Wynants, E.; Crauwels, S.; Verreth, C.; Gianotten, N.; Lievens, B.; Claes, J.; Van Campenhout, L. Microbial dynamics during production of lesser mealworms (Alphitobius diaperinus) for human consumption at industrial scale. Food Microbiol. 2018, 70, 181–191. [Google Scholar] [CrossRef]
- Jensen, L.D.; Miklos, R.; Dalsgaard, T.K.; Heckmann, L.H.; Nørgaard, J.V. Nutritional evaluation of common (Tenebrio molitor) and lesser (Alphitobius diaperinus) mealworms in rats and processing effect on the lesser mealworm. J. Insects Food Feed 2019, 5, 257–266. [Google Scholar] [CrossRef]
- Rumbos, C.I.; Karapanagiotidis, I.T.; Mente, E.; Psofakis, P.; Athanassiou, C.G. Evaluation of various commodities for the development of the yellow mealworm, Tenebrio molitor. Sci. Rep. 2020, 10, 11224. [Google Scholar] [CrossRef]
- Leni, G.; Soetemans, L.; Jacobs, J.; Depraetere, S.; Gianotten, N.; Bastiaens, L.; Caligiani, A.; Sforza, S. Protein hydrolysates from Alphitobius diaperinus and Hermetia illucens larvae treated with commercial proteases. J. Insects Food Feed. 2020, 6, 393–404. [Google Scholar] [CrossRef]
- Roncolini, A.; Milanović, V.; Aquilanti, L.; Cardinali, F.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Belleggia, L.; Pasquini, M.; Mozzon, M.; et al. Lesser mealworm (Alphitobius diaperinus) powder as a novel baking ingredient for manufacturing high-protein, mineral-dense snacks. Food Res. Int. 2020, 131, 109031. [Google Scholar] [CrossRef]
- Soetemans, L.; Gianotten, N.; Bastiaens, L. Agri-Food Side-Stream Inclusion in The Diet of Alphitobius Diaperinus. Part 2: Impact on Larvae Composition. Insects 2020, 11, 190. [Google Scholar] [CrossRef] [Green Version]
- McAllister, J.C.; Steelman, C.D.; Newberry, L.A.; Skeeles, J.K. Isolation of Infectious Bursal Disease Virus from the Lesser Mealworm, Alphitobius diaperinus (Panzer). Poult. Sci. 1995, 74, 45–49. [Google Scholar] [CrossRef]
- Retamales, J.; Vivallo, F.; Robeson, J. Insects associated with chicken manure in a breeder poultry farm of Central Chile. Arch. Med. Vet. 2011, 43, 79–83. [Google Scholar] [CrossRef]
- Eidson, C.S.; Schmittle, S.C.; Goode, R.B.; Lal, J.B. Induction of leukosis tumors with the beetle Alphitobius diaperinus. Am. J. Vet. Res. 1966, 27, 1053–1057. [Google Scholar] [PubMed]
- Snedeker, C.; Wills, F.K.; Moulthrop, I.M. Some Studies on the Infectious Bursal Agent. Avian Dis. 1967, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- De las Casas, E.R.; Harein, P.K.; Deshmukh, D.R.; Pomeroy, B.S. The relationship between the lesser mealworm and avian viruses. I. Reovirus 24. Environ. Entomol. 1973, 2, 1043–1047. [Google Scholar] [CrossRef]
- De Las Casas, E.; Harein, P.K.; Deshmukh, D.R.; Pomeroy, B.S. Relationship Between the Lesser Mealworm, Fowl Pox, and Newcastle Disease Virus in Poultry. J. Econ. Entomol. 1976, 69, 775–779. [Google Scholar] [CrossRef]
- Despins, J.L.; Axtell, R.C.; Rives, D.V.; Guy, J.S.; Ficken, M.D. Transmission of Enteric Pathogens of Turkeys by Darkling Beetle Larva (Alphitobius diaperinus). J. Appl. Poult. Res. 1994, 3, 61–65. [Google Scholar] [CrossRef]
- Goodwin, M.A.; Waltman, W.D. Transmission of Eimeria, Viruses, and Bacteria to Chicks: Darkling Beetles (Alphitobius diaperinus) as Vectors of Pathogens. J. Appl. Poult. Res. 1996, 5, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.W.; Guy, J.S.; Stringham, S.M. Limited Transmission of Turkey Coronavirus in Young Turkeys by Adult Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Med. Entomol. 2000, 37, 480–483. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.-C.; Giambrone, J.J.; Macklin, K.S. Detection of infectious laryngotracheitis virus from darkling beetles and their immature stage (lesser mealworms) by quantitative polymerase chain reaction and virus isolation. J. Appl. Poult. Res. 2012, 21, 33–38. [Google Scholar] [CrossRef]
- Li, Z.; Huang, S.; Huang, W.; Geng, H.; Zhao, Y.; Li, M.; Chen, Y.; Su, S. A scientific note on detection of honeybee viruses in the darkling beetle (Alphitobius diaperinus, Coleoptera: Tenebrionidae), a new pest in Apis cerana cerana colonies. Apidologie 2016, 47, 759–761. [Google Scholar] [CrossRef] [Green Version]
- Vandeweyer, D.; Lievens, B.; Van Campenhout, L. Identification of bacterial endospores and targeted detection of foodborne viruses in industrially reared insects for food. Nat. Food 2020, 1, 511–516. [Google Scholar] [CrossRef]
- Vigneron, A.; Jehan, C.; Rigaud, T.; Moret, Y. Immune Defenses of a Beneficial Pest: The Mealworm Beetle, Tenebrio molitor. Front. Physiol. 2019, 10, 138. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Nat. C J Biosci. 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Brandon, A.M.; Gao, S.H.; Tian, R.M.; Ning, D.L.; Yang, S.S.; Zhou, J.Z.; Wu, W.-M.; Criddle, C.S. Affiliations expand Biodegradation of polyethylene and plastic mixtures in mealworms (larvae of Tenebrio molitor) and effects on the gut microbiome. Environ. Sci. Technol. 2018, 52, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Gençer, D.; Yeşilyurt, A.; Güllü, M.; Demir, I.; Nalçacioğlu, R. Insecticidal activities of wild type and recombinant invertebrate iridescent viruses on five common pests. Türk. Entomol. Derg. 2020, 44, 365–373. [Google Scholar] [CrossRef]
- Kelly, D.C.; Ayres, M.D.; Lescott, T.; Robertson, J.S.; Happ, G.M. A Small Iridescent Virus (Type 29) Isolated from Tenebrio molitor: A Comparison of its Proteins and Antigens with Six Other Iridescent Viruses. J. Gen. Virol. 1979, 42, 95–105. [Google Scholar] [CrossRef]
- Black, P.N.; Blair, C.D.; Butcher, A.; Capinera, J.L.; Happ, G.M. Biochemistry and ultrastructure of iridescent virus type 29. J. Invertebr. Pathol. 1981, 38, 12–21. [Google Scholar] [CrossRef]
- Szelei, J.; Woodring, J.; Goettel, M.S.; Duke, G.; Jousset, F.-X.; Liu, K.Y.; Zadori, Z.; Li, Y.; Styer, E.; Boucias, D.G.; et al. Susceptibility of North-American and European crickets to Acheta domesticus densovirus (AdDNV) and associated epizootics. J. Invertebr. Pathol. 2011, 106, 394–399. [Google Scholar] [CrossRef]
- La Fauce, K.A.; Owens, L. The use of insects as a bioassay for Penaeus merguiensis densovirus (PmergDNV). J. Invertebr. Pathol. 2008, 98, 1–6. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Song, S.H.; Kim, N.J. Developmental characteristics of Zophobas atratus (Coleoptera: Tenebrionidae) larvae in different instars. Int. J. Indust. Entomol. 2015, 30, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Fursov, V.N.; Cherney, L.S. Zophobas atratus (Fabricius, 1775)—New genus and species of darkling beetles (Coleoptera, Tenebrionidae) for the fauna of Ukraine. Ukr. Entomol. J. 2018, 1, 10–24. [Google Scholar] [CrossRef]
- Park, H.C.; Jung, B.H.; Han, T.; Lee, Y.B.; Kim, S.-H.; Kim, N.J. Taxonomy of introduced commercial insect, Zophobas atratus (Coleoptera; Tenebrionidae) and a comparison of DNA barcoding with similar tenebrionids, Promethis valgipes and Tenebrio molitor in Korea. J. Sericult. Entomol. Sci. 2013, 51, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.R.S.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Santos, E.M. Nutritional composition of insects Gryllus assimilis and Zophobas morio: Potential foods harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Zaelor, J.; Kitthawee, S. Growth response to population density in larval stage of darkling beetles (Coleoptera; Tenebrionidae) Tenebrio molitor and Zophobas atratus. Agric. Nat. Res. 2018, 52, 603–606. [Google Scholar] [CrossRef]
- Tschinkel, W.R.; Willson, C.D. Inhibition of pupation due to crowding in some tenebrionid beetles. J. Exp. Zool. 1971, 176, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Quennedey, A.; Aribi, N.; Everaerts, C.; Delbecque, J.-P. Postembryonic development of Zophobas atratus Fab. (Coleoptera: Tenebrionidae) under crowded or isolated conditions and effects of juvenile hormone analogue applications. J. Insect Physiol. 1995, 41, 143–152. [Google Scholar] [CrossRef]
- Delbecque, J.-P.; Pitoizet, N.; Quennedey, A.; Aribi, N. Ecdysteroid titres in a tenebrionid beetle, Zophobas atratus: Effects of grouping and isolation. J. Insect Physiol. 1997, 43, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Forgach, P.; Marton, S.; Bercic, R.L.; Vida, L.; Rusvai, M. Identification of a novel densovirus in the darkling beetle Zophobas morio. Acta Microbiol. Immunol. Hungar. 2015, 62, 130–131. [Google Scholar] [CrossRef]
- Tokarev, Y.S.; Malysh, S.M.; Volodartseva, Y.V.; Gerus, A.V.; Berezin, M.V. Molecular Identification of a Densovirus in Healthy and Diseased Zophobas morio (Coleoptera, Tenebrionidae). Intervirology 2019, 62, 222–226. [Google Scholar] [CrossRef]
- Yang, W.T.; Shi, S.H.; Jiang, Y.L.; Zhao, L.; Chen, H.L.; Huang, K.Y.; Yang, G.-L.; Wang, C.-F. Genetic characterization of a densovirus isolated from great tit (Parus major) in China. Infect. Genet. Evol. 2016, 41, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Oppert, B.; Perkin, L.C.; Lorenzen, M.; Dossey, A.T. Transcriptome analysis of life stages of the house cricket, Acheta domesticus, to improve insect crop production. Sci. Rep. 2020, 10, 3471. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (Acheta domesticus). EFSA J. 2018, 16, e16082. [Google Scholar] [CrossRef]
- Osimani, A.; Milanović, V.; Cardinali, F.; Roncolini, A.; Garofalo, C.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; Raffaelli, N.; et al. Bread enriched with cricket powder (Acheta domesticus): A technological, microbiological and nutritional evaluation. Innov. Food Sci. Emerg. Technol. 2018, 48, 150–163. [Google Scholar] [CrossRef]
- Udomsil, N.; Imsoonthornruksa, S.; Gosalawit, C.; Ketudat-Cairns, M. Nutritional Values and Functional Properties of House Cricket (Acheta domesticus) and Field Cricket (Gryllus bimaculatus). Food Sci. Technol. Res. 2019, 25, 597–605. [Google Scholar] [CrossRef]
- Orkusz, A. Edible Insects versus Meat—Nutritional Comparison: Knowledge of Their Composition Is the Key to Good Health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Granberg, F.; Low, M.; Onorati, P.; Semberg, E.; Jansson, A.; Berggren, Å. Virus Diversity and Loads in Crickets Reared for Feed: Implications for Husbandry. Front. Vet. Sci. 2021, 8, 642085. [Google Scholar] [CrossRef] [PubMed]
- Styer, E.L.; Hamm, J.J. Report of a densovirus in a commercial cricket operation in the southeastern United States. J. Invertebr. Pathol. 1991, 58, 283–285. [Google Scholar] [CrossRef]
- Liu, K.; Li, Y.; Jousset, F.-X.; Zadori, Z.; Szelei, J.; Yu, Q.; Tijssen, P. The Acheta domesticus Densovirus, Isolated from the European House Cricket, Has Evolved an Expression Strategy Unique among Parvoviruses. J. Virol. 2011, 85, 10069–10078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, H.T.; Iwao, H.; Szelei, J.; Li, Y.; Liu, K.; Bergoin, M.; Tijssen, P. Comparative Genomic Analysis of Acheta domesticus Densovirus Isolates from Different Outbreaks in Europe, North America, and Japan. Genome Announc. 2013, 1, e00629-13. [Google Scholar] [CrossRef] [Green Version]
- Meynadier, G.; Matz, G.; Veyrunes, J.-C.; Bres, N. Virus de type densonucléose chez les orthoptères. Ann. Soc. Entomol. Fr. 1977, 13, 487–493. [Google Scholar]
- Pham, H.T.; Bergoin, M.; Tijssen, P. Acheta domesticus volvovirus, a novel single-stranded circular DNA virus of the house cricket. Genome Announc. 2013, 1, e00079-13. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.T.; Iwao, H.; Bergoin, M.; Tijssen, P. New Volvovirus Isolates from Acheta domesticus (Japan) and Gryllus assimilis (United States). Genome Announc. 2013, 1, e00328-13. [Google Scholar] [CrossRef] [Green Version]
- Pham, H.T.; Yu, Q.; Bergoin, M.; Tijssen, P. A Novel Ambisense Densovirus, Acheta domesticus Mini Ambidensovirus, from Crickets. Genome Announc. 2013, 1, e00914-13. [Google Scholar] [CrossRef] [Green Version]
- De Miranda, J.R.; Granberg, F.; Onorati, P.; Jansson, A.; Berggren, Å. Virus Prospecting in Crickets—Discovery and Strain Divergence of a Novel Iflavirus in Wild and Cultivated Acheta domesticus. Viruses 2021, 13, 364. [Google Scholar] [CrossRef]
- Kleespies, R.; Tidona, C.; Darai, G. Characterization of a New Iridovirus Isolated from Crickets and Investigations on the Host Range. J. Invert. Pathol. 1999, 73, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Plus, N.; Scotti, P.D. The biological properties of eight different isolates of cricket paralysis virus. Ann. Inst. Pasteur/Virol. 1984, 135, 257–268. [Google Scholar] [CrossRef]
- Christian, P.D.; Scotti, P. A Suggested Taxonomy and Nomenclature for the Cricket Paralysis and Drosophila C Virus Complex. J. Invert. Pathol. 1994, 63, 157–162. [Google Scholar] [CrossRef]
- Lee, Y.; Fuxa, J.R. Transport of Wild-Type and Recombinant Nucleopolyhedroviruses by Scavenging and Predatory Arthropods. Microb. Ecol. 2000, 39, 301–313. [Google Scholar] [CrossRef]
- Lee, Y.; Fuxa, J.R. Ingestion and Defecation of Recombinant and Wild-Type Nucleopolyhedroviruses by Scavenging and Predatory Arthropods. Environ. Entomol. 2000, 29, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Valles, S.M.; Chen, Y. Serendipitous discovery of an RNA virus from the cricket, Acheta domesticus. Fla. Entomol. 2006, 89, 282–283. [Google Scholar] [CrossRef]
- Porter, S.D.; Valles, S.M.; Pereira, R.M. Scavenging crickets (Orthoptera: Gryllidae) transmit Solenopsis invicta virus 3 to red imported fire ant (Hymenoptera: Formicidae) colonies. Fla. Entomol. 2016, 99, 811–881. [Google Scholar] [CrossRef]
- La Fauce, K.A.; Owens, L. RNA interference reduces PmergDNV expression and replication in an in vivo cricket model. J. Invert. Pathol. 2009, 100, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Sakuna, K.; Elliman, J.; Owens, L. Assessment of a cricket, Acheta domesticus, bioassay for Chequa Iflavirus and bunya-like virus from redclaw crayfish Cherax quadricarinatus. J. Invert. Pathol. 2017, 150, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; Van Campenhout, L. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int. J. Food Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef]
- Vandeweyer, D.; Wynants, E.; Crauwels, S.; Verreth, C.; Viaene, N.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial Dynamics during Industrial Rearing, Processing, and Storage of Tropical House Crickets (Gryllodes sigillatus) for Human Consumption. Appl. Environ. Microbiol. 2018, 84, e00255-18. [Google Scholar] [CrossRef] [Green Version]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional properties of tropical banded cricket (Gryllodes sigillatus) protein hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Otte, D.; Cade, W. African Crickets (Gryllidae). 6. The Genus Gryllus and Some Related Genera (Gryllinae, Gryllini). Proc. Nat. Acad. Sci. Phila. 1984, 136, 98–122. [Google Scholar]
- Ghosh, S.; Lee, S.-M.; Jung, C.; Meyer-Rochow, V.B. Nutritional composition of five commercial edible insects in South Korea. J. Asia-Pac. Entomol. 2017, 20, 686–694. [Google Scholar] [CrossRef]
- Chae, K.-S.; Shin, C.-S.; Shin, W.-S. Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Sci. Biotechnol. 2018, 27, 631–639. [Google Scholar] [CrossRef]
- Merkel, G. The effects of temperature and food quality on the larval development of Gryllus bimaculatus (Orthoptera, Gryllidae). Oecologia 1977, 30, 129–140. [Google Scholar] [CrossRef]
- Sorjonen, J.M.; Valtonen, A.; Hirvisalo, E.; Karhapää, M.; Lehtovaara, V.J.; Lindgren, J.; Roininen, H. The plant-based by-product diets for the mass-rearing of Acheta domesticus and Gryllus bimaculatus. PLoS ONE 2019, 14, e0218830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobermann, D.; Michaelson, L.; Field, L.M. The effect of an initial high-quality feeding regime on the survival of Gryllus bimaculatus (black cricket) on bio-waste. J. Insects Food Feed. 2019, 5, 117–123. [Google Scholar] [CrossRef]
- Grabowski, N.T.; Klein, G. Microbiology of cooked and dried edible Mediterranean field crickets (Gryllus bimaculatus) and superworms (Zophobas atratus) submitted to four different heating treatments. Food Sci. Technol. Int. 2016, 23, 17–23. [Google Scholar] [CrossRef]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, J.H. The edible insect Gryllus bimaculatus protects against gut-derived inflammatory responses and liver damage in mice after acute alcohol exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, B.-J.; You, S.-G.; Lee, S.-M. 2021. Nutritional evaluation of cricket, Gryllus bimaculatus, meal as fish meal substitute for olive flounder, Paralichthys olivaceus, juveniles. J. World Aquac. Soc. 2021, 52, 859–880. [Google Scholar] [CrossRef]
- Just, F.; Essbauer, S.; Ahne, W.; Blahak, S. Occurrence of an Invertebrate Iridescent-Like Virus (Iridoviridae) in Reptiles. J. Vet. Med. B 2001, 48, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Papp, T.; Spann, D.; Marschang, R.E. Development and use of a real-time polymerase chain reaction for the detection of Group II Invertebrate Iridoviruses in pet lizards and prey insects. J. Zoo Wildl. Med. 2014, 45, 219–227. [Google Scholar] [CrossRef]
- Huger, A.M. A new virus disease of crickets (Orthoptera: Gryllidae) causing macronucleosis of fatbody. J. Invertebr. Pathol. 1985, 45, 108–111. [Google Scholar] [CrossRef]
- Wang, Y.; Kleespies, R.G.; Huger, A.M.; Jehle, J.A. The Genome of Gryllus bimaculatus Nudivirus Indicates an Ancient Diversification of Baculovirus-Related Nonoccluded Nudiviruses of Insects. J. Virol. 2007, 81, 5395–5406. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jehle, J.A. Nudiviruses and other large, double-stranded circular DNA viruses of invertebrates: New insights on an old topic. J. Invertebr. Pathol. 2009, 101, 187–193. [Google Scholar] [CrossRef]
- Scotti, P.D.; Longworth, J.F.; Plus, N.; Croizier, G.; Reinganum, C. The Biology and Ecology of Strains of an Insect Small RNA Virus Complex. Adv. Vir. Res. 1981, 26, 117–143. [Google Scholar] [CrossRef]
- Purrini, K.; Kohring, G.-W.; Seguni, Z. Studies on a new disease in a natural population of migratory locusts, Locusta migratoria, caused by an entomopoxvirus. J. Invertebr. Pathol. 1988, 51, 284–286. [Google Scholar] [CrossRef]
- Levin, D.B.; Adachi, D.; Williams, L.L.; Myles, T.G. Host Specificity and Molecular Characterization of the Entomopoxvirus of the Lesser Migratory Grasshopper, Melanoplus sanguinipes. J. Invertebr. Pathol. 1993, 62, 241–247. [Google Scholar] [CrossRef]
- Jaeger, B.; Langridge, W.H.R. Infection of Locusta migratoria with entomopoxviruses from Arphia conspersa and Melanoplus sanguinipes grasshoppers. J. Invertebr. Pathol. 1984, 43, 374–382. [Google Scholar] [CrossRef]
- Streett, D.; Woods, S.; Erlandson, M. Entomopoxviruses of grasshoppers and locusts: Biology and biological control potential. Mem. Ent. Soc. Can. 1997, 129, 115–130. [Google Scholar] [CrossRef]
- Faktor, O.; Raviv, D. A polymerase chain reaction for the detection of nucleopolyhedroviruses in infected insects: The fate of the Spodoptera littoralis virus in Locusta migratoria. J. Virol. Methods 1996, 61, 95–101. [Google Scholar] [CrossRef]
- Bensimon, A.; Zinger, S.; Gerassi, E.; Hauschner, A.; Harpaz, I.; Sela, I. “Dark cheeks,” a lethal disease of locusts provoked by a lepidopterous baculovirus. J. Invertebr. Pathol. 1987, 50, 254–260. [Google Scholar] [CrossRef]
- Cologan, D.J. Studies of the mortality of Locusta migratoria (L.) treated with a polyhedrosis virus from the grasshopper Caledia captiva (F.) (Orthoptera: Acrididae). Bull. Entomol. Res. 1986, 76, 539. [Google Scholar] [CrossRef]
- Hawkes, F. A virus-like structure in the desert locust. Naturwissenschaften 1968, 55, 547. [Google Scholar] [CrossRef]
- Purrini, K.; Rohde, M. Light and electron microscope studies on two new diseases in natural populations of the desert locust, Schistocerca gregaria, and the grassland locust, Chortipes sp., caused by two entomopoxviruses. J. Invertebr. Pathol. 1988, 51, 281–283. [Google Scholar] [CrossRef]
- Capinera, J.L.; Squitier, J.M. American Grasshopper, Schistocerca americana (Drury) (Insecta: Orthoptera: Acrididae); IFAS Extension, University of Florida: Gainesville, FL, USA, 2017. [Google Scholar] [CrossRef]
- Henry, J.E.; Jutila, J.W. The isolation of a polyhedrosis virus from a grasshopper. J. Invert. Pathol. 1966, 8, 417–418. [Google Scholar] [CrossRef]
- Streett, D.A.; Oma, E.A.; Henry, J.E. Cross infection of three grasshopper species with the Melanoplus sanguinipes entomopoxvirus. J. Invertebr. Pathol. 1990, 56, 419–421. [Google Scholar] [CrossRef]
- Henry, J.E. Development of the crystalline-array virus (CAV) in cultures of dorsal vessels from Schistocerca americana (Orthoptera: Acrididae). J. Invertebr. Pathol. 1972, 19, 325–330. [Google Scholar] [CrossRef]
- Henry, J.E.; Oma, E.A. Ultrastructure of the replication of the grasshopper crystalline-array virus in Schistocerca americana compared with other picornaviruses. J. Invertebr. Pathol. 1973, 21, 273–281. [Google Scholar] [CrossRef]
- Semberg, E.; de Miranda, R.J.; Low, M.; Jansson, A.; Forsgren, E.; Berggren, Å. Diagnostic protocols for the detection of Acheta domesticus densovirus (AdDV) in cricket frass. J. Virol. Methods 2019, 264, 61–64. [Google Scholar] [CrossRef]
- Martínez, G.; Christian, P.; Marina, C.; Williams, T. Sensitivity of Invertebrate iridescent virus 6 to organic solvents, detergents, enzymes and temperature treatment. Virus Res. 2003, 91, 249–254. [Google Scholar] [CrossRef]
- Spranghers, T.; Noyez, A.; Schildermans, K.; De Clercq, P. Cold Hardiness of the Black Soldier Fly (Diptera: Stratiomyidae). J. Econom. Entomol. 2017, 110, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I). Renew. Energ. 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Da Silva, G.D.P.; Hesselberg, T. A Review of the Use of Black Soldier Fly Larvae, Hermetia illucens (Diptera: Stratiomyidae), to Compost Organic Waste in Tropical Regions. Neotrop. Entomol. 2019, 49, 151–162. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Shafi, M.E.; Alghamdi, W.Y.; Abdelnour, S.A.; Shehata, A.M.; Noreldin, A.E.; Ashour, E.A.; Swelum, A.A.; Al-Sagan, A.A.; Alkhateeb, M.; et al. Black Soldier Fly (Hermetia illucens) Meal as A Promising Feed Ingredient for Poultry: A Comprehensive Review. Agriculture 2020, 10, 339. [Google Scholar] [CrossRef]
- English, G.; Wanger, G.; Colombo, S.M. A review of advancements in black soldier fly (Hermetia illucens) production for dietary inclusion in salmonid feeds. J. Agr. Food Res. 2021, 5, 100164. [Google Scholar] [CrossRef]
- Kim, W.; Bae, S.; Park, K.; Lee, S.; Choi, Y.; Han, S.; Koh, Y. Biochemical characterization of digestive enzymes in the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). J. Asia-Pac. Entomol. 2011, 14, 11–14. [Google Scholar] [CrossRef]
- Gougbedji, A.; Agbohessou, P.; Lalèyè, P.A.; Francis, F.; Megido, R.C. Technical basis for the small-scale production of black soldier fly, Hermetia illucens (L. 1758), meal as fish feed in Benin. J. Agr. Food Res. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agr. 2016, 97, 2594–2600. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—a review. J. Insects Food Feed. 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on Different Organic By-Products: Influence on Growth, Waste Reduction, and Environmental Impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef] [Green Version]
- Ewusie, E.A.; Kwapong, P.K.; Ofosu-Budu, G.; Sandrock, C.; Stamer, A.; Akumah, A.; Adamtey, N. The black soldier fly, Hermetia illucens (Diptera: Stratiomyidae): Trapping and culturing of wild colonies in Ghana. Sci. Afr. 2019, 5, e00134. [Google Scholar] [CrossRef]
- Scala, A.; Cammack, J.A.; Salvia, R.; Scieuzo, C.; Franco, A.; Bufo, S.A.; Falabella, P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci. Rep. 2020, 10, 19448. [Google Scholar] [CrossRef]
- Gligorescu, A.; Christian, H.F.; Larsen, P.F.; Nørgaard, J.V.; Lars-Henrik, L.H. Production and Optimization of Hermetia illucens (L.) Larvae Reared on Food Waste and Utilized as Feed Ingredient. Sustainability 2020, 12, 9864. [Google Scholar] [CrossRef]
- Joosten, L.; Lecocq, A.; Jensen, A.B.; Haenen, O.; Schmitt, E.; Eilenberg, J. Review of insect pathogen risks for the black soldier fly (Hermetia illucens) and guidelines for reliable production. Entomol. Exp. Appl. 2020, 168, 432–447. [Google Scholar] [CrossRef]
- Lalander, C.H.; Fidjeland, J.; Diener, S.; Eriksson, S.; Vinnerås, B. High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron. Sustain. Dev. 2014, 35, 261–271. [Google Scholar] [CrossRef]
- Khamesipour, F.; Lankarani, K.B.; Honarvar, B.; Kwenti, T.E. A systematic review of human pathogens carried by the housefly (Musca domestica L.). BMC Public Health 2018, 18, 1049. [Google Scholar] [CrossRef] [PubMed]
- Stoffolano, J.G. Fly foregut and transmission of microbes. Adv. Insect Phys. 2019, 57, 27–95. [Google Scholar] [CrossRef]
- Förster, M.; Klimpel, S.; Sievert, K. The house fly (Musca domestica) as a potential vector of metazoan parasites caught in a pig-pen in Germany. Vet. Parasitol. 2009, 160, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.; Molnar, C.; Morley, F.; Wray, T.; Robinson, F.; Khan, B.; Goonewardene, L. Nutritive value of house fly (Musca domestica) larvae as a feed supplement for turkey poults. Anim. Feed Sci. Technol. 2003, 105, 225–230. [Google Scholar] [CrossRef]
- Koné, N.; Sylla, M.; Nacambo, S.; Kenis, M. Production of house fly larvae for animal feed through natural oviposition. J. Insects Food Feed. 2017, 3, 177–186. [Google Scholar] [CrossRef]
- Fitches, E.C.; Dickinson, M.; De Marzo, D.; Wakefield, M.E.; Charlton, A.C.; Hall, H. Alternative protein production for animal feed: Musca domestica productivity on poultry litter and nutritional quality of processed larval meals. J. Insects Food Feed. 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Ganda, H.; Abihona, H.A.; Zannou-Boukari, E.T.; Kenis, M.; Chrysostome, C.A.A.M.; Mensah, G.A. Influence of adult diet on biological parameters of the housefly, Musca domestica L. (Diptera: Muscidae). J. Basic Appl. Zool. 2020, 81, 46–53. [Google Scholar] [CrossRef]
- Čičková, H.; Pastor, B.; Kozánek, M.; Martínez-Sánchez, A.; Rojo, S.; Takáč, P. Biodegradation of pig manure by the housefly, Musca domestica: A viable ecological strategy for pig manure management. PLoS ONE 2012, 7, e32798. [Google Scholar] [CrossRef] [Green Version]
- Čičková, H.; Newton, G.L.; Lacy, R.C.; Kozánek, M. The use of fly larvae for organic waste treatment. J. Waste Manag. 2015, 35, 68–80. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Z.; Czapar, G.F.; Winkler, M.K.; Zheng, J. A full-scale house fly (Diptera: Muscidae) larvae bioconversion system for value-added swine manure reduction. Waste Manag. Res. 2013, 31, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Yu, L.; Li, H.; Xu, X.; Yang, Z. Use of housefly (Musca domestica L.) larvae to bioconversion food waste for animal nutrition and organic fertilizer. Environ. Sci. Pollut. Res. 2021, 28, 48921–48928. [Google Scholar] [CrossRef] [PubMed]
- Cortes Ortiz, J.A.; Ruiz, A.T.; Morales-Ramos, J.A.; Thomas, M.; Rojas, M.G.; Tomberlin, J.K.; Jullien, R.L. Insect Mass Production Technologies. In Insects as Sustainable Food Ingredients: Production, Processing and Food Applications; Morales-Ramos, J.A., Thomas, M., Rojas, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–201. [Google Scholar] [CrossRef]
- Moussa, A.Y. A new virus disease in the housefly, Musca domestica (Diptera). J. Invertebr. Pathol. 1978, 31, 204–216. [Google Scholar] [CrossRef]
- Coler, R.R.; Boucias, D.G.; Frank, J.H.; Maruniak, J.E.; Garcia-Canedo, A.; Pendland, J.C. Characterization and description of a virus causing salivary gland hyperplasia in the housefly, Musca domestica. Med. Vet. Entomol. 1993, 7, 275–282. [Google Scholar] [CrossRef]
- Graczyk, T.K.; Knight, R.; Gilman, R.H.; Cranfield, M.R. The role of non-biting flies in the epidemiology of human infectious diseases. Microbes Infect. 2001, 3, 231–235. [Google Scholar] [CrossRef]
- Noguchi, H. The relation of mosquitoes and flies to the epidemiology of acute poliomyelitis. J. Exp. Med. 1917, 26, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gudnadottir, M.G. Studies of the fate of type 1 polioviruses in flies. J. Exp. Med. 1961, 113, 159–176. [Google Scholar] [CrossRef] [Green Version]
- Hurlbut, H.S. The Recovery of Poliomyelitis Virus after Parenteral Introduction into Cockroaches and Houseflies. J. Inf. Dis. 1950, 86, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.W. Experiments on insect transmission of the virus of poliomyelitis. J. Exp. Med. 1912, 16, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Melnick, J.L. The survival of poliomyelitis and coxsackie viruses following their ingestion by flies. J. Exp. Med. 1952, 96, 255–271. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, S.B.; Nakao, J.C.; Beran, G.W. Human enteroviruses in animals and arthropods in the central Philippines. Southeast Asian J. Trop. Med. Public Health 1972, 3, 45–51. [Google Scholar] [PubMed]
- Tan, S.W.; Yap, K.L.; Lee, H.L. Mechanical Transport of Rotavirus by the Legs and Wings of Musca domestica (Diptera: Muscidae). J. Med. Entomol. 1997, 34, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Haddow, A.D.; Nasar, F.; Schellhase, C.W.; Moon, R.D.; Padilla, S.L.; Zeng, X.; Trefry, J.C. Low potential for mechanical transmission of Ebola virus via house flies (Musca domestica). Parasit. Vectors. 2017, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Prompiboon, P.; Lietze, V.-U.; Denton, J.S.S.; Geden, C.J.; Steenberg, T.; Boucias, D.G. Musca domestica Salivary Gland Hypertrophy Virus, a Globally Distributed Insect Virus That Infects and Sterilizes Female Houseflies. Appl. Environ. Microbiol. 2009, 76, 994–998. [Google Scholar] [CrossRef] [Green Version]
- Geden, C.J.; Lietze, V.-U.; Boucias, D.G. Seasonal Prevalence and Transmission of Salivary Gland Hypertrophy Virus of House Flies (Diptera: Muscidae). J. Med. Entomol. 2008, 45, 42–51. [Google Scholar] [CrossRef]
- Geden, C.J.; Steenberg, T.; Lietze, V.-U.; Boucias, D.G. Salivary gland hypertrophy virus of house flies in Denmark: Prevalence, host range, and comparison with a Florida isolate. J. Vector Ecol. 2011, 36, 231–238. [Google Scholar] [CrossRef]
- Geden, C.; Garcia-Maruniak, A.; Lietze, V.U.; Maruniak, J.; Boucias, D.G. Impact of House Fly Salivary Gland Hypertrophy Virus (MdSGHV) on a Heterologous Host, Stomoxys calcitrans. J. Med. Entomol. 2011, 48, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.-U.; Geden, C.J.; Blackburn, P.; Boucias, D.G. Effects of Salivary Gland Hypertrophy Virus on the Reproductive Behavior of the Housefly, Musca domestica. Appl. Environ. Microbiol. 2007, 73, 6811–6818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lietze, V.-U.; Abd-Alla, A.M.M.; Boucias, D.G. Two hytrosaviruses, MdSGHV and GpSGHV, induce distinct cytopathologies in their respective host insects. J. Invertebr. Pathol. 2011, 107, 161–163. [Google Scholar] [CrossRef]
- Lietze, V.-U.; Salem, T.Z.; Prompiboon, P.; Boucias, D.G. Tissue tropism of the Musca domestica salivary gland hypertrophy virus. Virus Res. 2011, 155, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lietze, V.-U.; Sims, K.R.; Salem, T.Z.; Geden, C.J.; Boucias, D.G. Transmission of MdSGHV among adult house flies, Musca domestica (Diptera: Muscidae), occurs via oral secretions and excreta. J. Invertebr. Pathol. 2009, 101, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lietze, V.-U.; Geden, C.J.; Doyle, M.A.; Boucias, D.G. Disease Dynamics and Persistence of Musca domestica Salivary Gland Hypertrophy Virus Infections in Laboratory House Fly (Musca domestica) Populations. Appl. Environ. Microbiol. 2011, 78, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Kariithi, H.M.; Yao, X.; Yu, F.; Teal, P.E.; Verhoeven, C.P.; Boucias, D.G. Responses of the Housefly, Musca domestica, to the Hytrosavirus Replication: Impacts on Host’s Vitellogenesis and Immunity. Front. Microbiol. 2017, 8, 583. [Google Scholar] [CrossRef] [Green Version]
- Schaler, J.; Stoffolano, J.; Fausto, A.M.; Gambellini, G.; Burand, J. Effect of diet on adult house fly (Diptera: Muscidae) injected with the Salivary Gland Hypertrophy Virus (MdSGHV). J. Insect Sci. 2018, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Rachimi, S.; Burand, J.P.; Geden, C.; Stoffolano, J.G. The Effect of the Musca domestica Salivary Gland Hypertrophy Virus on Food Consumption in Its Adult Host, the Common House Fly (Diptera: Muscidae). J. Med. Entomol. 2021, 58, 1398–1404. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Hawkes, R.A.; Dickson, M.R.; Shipp, E.; Woods, A. Serological relationships of the housefly virus and some members of the family Reoviridae. Aust. J. Biol. Sci. 1982, 35, 669–678. [Google Scholar] [CrossRef]
- Wanaratana, S.; Panyim, S.; Pakpinyo, S. The potential of house flies to act as a vector of avian influenza subtype H5N1 under experimental conditions. Med. Vet. Entomol. 2010, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Wanaratana, S.; Amonsin, A.; Chaisingh, A.; Panyim, S.; Sasipreeyajan, J.; Pakpinyo, S. Experimental Assessment of Houseflies as Vectors in Avian Influenza Subtype H5N1 Transmission in Chickens. Avian Dis. 2013, 57, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wuryastuty, H.; Wasito, R. Molecular Identification of Avian Influenza A Virus in House Flies (Musca domestica Linnaeus) Collected from Different Poultry Farms in Indonesia. J. Sain Vet. 2013, 31, 1–7. [Google Scholar] [CrossRef]
- Tyasasmaya, T.; Wuryastuty, H.; Wasito, W.; Sievert, K. Avian Influenza Virus H5N1 Remained Exist in Gastrointestinal Tracts of House Flies 24 Hours Post-infection. Biology 2016, 17, 205–210. Available online: https://ojs.unud.ac.id/index.php/jvet/article/view/22119 (accessed on 29 October 2021).
- Salamatian, I.; Moshaverinia, A.; Razmyar, J.; Ghaemi, M. In vitro Acquisition and Retention of Low-Pathogenic Avian Influenza H9N2 by Musca domestica (Diptera: Muscidae). J. Med. Entomol. 2019, 57, 563–567. [Google Scholar] [CrossRef]
- Calibeo-Hayes, D.; Denning, S.S.; Stringham, S.M.; Guy, J.S.; Smith, L.G.; Watson, D.W. Mechanical Transmission of Turkey Coronavirus by Domestic Houseflies (Musca domestica Linnaeaus). Avian Dis. 2003, 47, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Rogoff, W.M.; Carbrey, E.G.; Bram, R.A.; Clark, T.B.; Gretz, G.H. Transmission of Newcastle Disease Virus by Insects: Detection in Wild Fannia Spp. (Diptera: Muscidae). J. Med. Entomol. 1975, 12, 225–227. [Google Scholar] [CrossRef]
- Watson, D.W.; Niño, E.L.; Rochon, K.; Denning, S.; Smith, L.; Guy, J.S. Experimental Evaluation of Musca domestica (Diptera: Muscidae) as a Vector of Newcastle Disease Virus. J. Med. Entomol. 2007, 44, 666–671. [Google Scholar] [CrossRef]
- Chakrabarti, S.; King, D.J.; Afonso, C.; Swayne, D.; Cardona, C.J.; Kuney, D.R.; Gerry, A.C. Detection and Isolation of Exotic Newcastle Disease Virus from Field-Collected Flies. J. Med. Entomol. 2007, 44, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; King, D.J.; Cardona, C.J.; Gerry, A.C. Persistence of Exotic Newcastle Disease Virus (ENDV) in Laboratory Infected Musca domestica and Fannia canicularis. Avian Dis. 2008, 52, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Barin, A.; Arabkhazaeli, F.; Rahbari, S.; Madani, S.A. The housefly, Musca domestica, as a possible mechanical vector of Newcastle disease virus in the laboratory and field. Med. Vet. Entomol. 2010, 24, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.; Braverman, Y. Insect Contribution to Horizontal Transmission of Reticuloendotheliosis virus. J. Med. Entomol. 2005, 42, 128–133. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Farnham, M.; Pijoan, C. Survival of porcine reproductive and respiratory syndrome virus in houseflies. Can. J. Vet. Res. 2003, 67, 198–203. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC227053/ (accessed on 28 October 2021).
- Otake, S.; Dee, S.A.; Rossow, K.D.; Moon, R.D.; Trincado, C.; Pijoan, C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica). Vet. Rec. 2003, 152, 73–76. [Google Scholar] [CrossRef]
- Otake, S.; Dee, S.A.; Moon, R.D.; Rossow, K.D.; Trincado, C.; Pijoan, C. Studies on the carriage and transmission of porcine reproductive and respiratory syndrome virus by individual houseflies (Musca domestica). Vet. Rec. 2004, 154, 80–85. [Google Scholar] [CrossRef]
- Schurrer, J.A.; Dee, S.A.; Moon, R.D.; Murtaugh, M.P.; Finnegan, C.P.; Deen, J.; Pijoan, C.B.J. Retention of ingested porcine reproductive and respiratory syndrome virus in houseflies. Am. J. Vet. Res. 2005, 66, 1517–1525. [Google Scholar] [CrossRef]
- Pitkin, A.; Deen, J.; Otake, S.; Moon, R.; Dee, S. Further assessment of houseflies (Musca domestica) as vectors for the mechanical transport and transmission of porcine reproductive and respiratory syndrome virus under field conditions. Can. J. Vet. Res. 2009, 73, 91–96. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC2666325/ (accessed on 28 October 2021).
- Blunt, R.; McOrist, S.; McKillen, J.; McNair, I.; Jiang, T.; Mellits, K. House fly vector for porcine circovirus 2b on commercial pig farms. Vet. Microbiol. 2011, 149, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Masiuk, D.N.; Nedzvetsky, V.S.; Sosnitskiy, A.I.; Kokarev, A.V.; Koliada, S.G. The characteristics, emergent properties and manner of spread in Ukraine of the Porcine Epidemic Diarrhea Virus. Reg. Mech. Biosyst. 2018, 9, 401–408. [Google Scholar] [CrossRef]
- Herm, R.; Tummeleht, L.; Jürison, M.; Vilem, A.; Viltrop, A. Trace amounts of African swine fever virus DNA detected in insects collected from an infected pig farm in Estonia. Vet. Med. Sci. 2019, 6, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Turčinavičienė, J.; Petrašiūnas, A.; Bernotienė, R.; Masiulis, M.; Jonušaitis, V. The contribution of insects to African swine fever virus dispersal: Data from domestic pig farms in Lithuania. Med. Vet. Entomol. 2020, 35, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Medveczky, I.; Kovács, L.; Kovács Sz, F.; Papp, L. The role of the housefly, Musca domestica, in the spread of Aujeszky’s disease (pseudorabies). Med. Vet. Entomol. 1988, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.R.; Mohr, K.A.; Clement, T.; Hain, K.S.; Myers, B.; Yaros, J.; Diel, D.G. Detection of the Emerging Picornavirus Senecavirus A in Pigs, Mice, and Houseflies. J. Clin. Microbiol. 2016, 54, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Turell, M.J.; Dohm, D.J.; Geden, C.J.; Hogsette, J.A.; Linthicum, K.J. Potential for Stable Flies and House Flies (Diptera: Muscidae) to Transmit Rift Valley Fever Virus1. J. Am. Mosq. Control Assoc. 2010, 26, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Bouillant, A.; Lee, V.H.; Hanson, R.P. Epizootiology on mink enteritis. II. Musca domestica L. as a possible vector of virus. Can. J. Comp. Med. Vet. Sci. 1965, 29, 148–152. [Google Scholar]
- Prieto, A.; Díaz-Cao, J.M.; Fernández-Antonio, R.; Panadero, R.; López-Lorenzo, G.; Díaz, P.; Fernández, G. Lesser housefly (Fannia canicularis) as possible mechanical vector for Aleutian mink disease virus. Vet. Microbiol. 2018, 221, 90–93. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Prutnikov, P.; Kononov, A. Detection of vaccine-like lumpy skin disease virus in cattle and Musca domestica L. flies in an outbreak of lumpy skin disease in Russia in 2017. Transbound. Emerg. Dis. 2018, 65, 1137–1144. [Google Scholar] [CrossRef]
- Sprygin, A.; Pestova, Y.; Wallace, D.B.; Tuppurainen, E.; Kononov, A.V. Transmission of lumpy skin disease virus: A short review. Virus Res. 2019, 269, 197637. [Google Scholar] [CrossRef] [PubMed]
- Boucias, D.; Baniszewski, J.; Prompiboon, P.; Lietze, V.; Geden, C. Enhancement of the Musca domestica hytrosavirus infection with orally delivered reducing agents. J. Invertebr. Pathol. 2015, 124, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.D.; Graham, J.R.; Mortensen, A. Standard methods for wax moth research. J. Apic. Res. 2013, 52, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.L. Pests and Pathogens of the Honeybee (Apis Mellifera L.) in Fiji. J. Apic. Res. 1990, 29, 53–59. [Google Scholar] [CrossRef]
- Malfroy, S.F.; Roberts, J.M.K.; Perrone, S.; Maynard, G.; Chapman, N. A pest and disease survey of the isolated Norfolk Island honey bee (Apis mellifera) population. J. Apic. Res. 2016, 55, 202–211. [Google Scholar] [CrossRef]
- Kwadha, C.A.; Ongamo, G.O.; Ndegwa, P.N.; Raina, S.K.; Fombong, A.T. The Biology and Control of the Greater Wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; Ansari, J.M.; Al Ghamdi, A.; Mohamed, O.M.; Kaur, M. Effect of larval nutrition on the development and mortality of Galleria mellonella (Lepidoptera: Pyralidae). Rev. Colomb. Entomol. 2014, 40, 49–54. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-04882014000100009 (accessed on 28 October 2021).
- Jorjão, A.L.; Oliveira, L.D.; Scorzoni, L.; Figueiredo-Godoi, L.M.A.; Cristina, A.; Prata, M.; Jorge, A.O.C.; Junqueira, J.C. From moths to caterpillars: Ideal conditions for Galleria mellonella rearing for in vivo microbiological studies. Virulence 2018, 9, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Khan, M.S. Study of the life cycle of greater wax moth (Galleria mellonella) under storage conditions in relation to different weather conditions. J. Entomol. Zool. Stud. 2018, 6, 444–447. Available online: https://www.entomoljournal.com/archives/2018/vol6issue3/PartG/6-2-76-351.pdf (accessed on 28 October 2021).
- Sohail, M.; Aqueel, M.A.; Dai, P.; Ellis, J.D.; Johnson, R. The Larvicidal and Adulticidal Effects of Selected Plant Essential Oil Constituents on Greater Wax Moths. J. Econom. Entomol. 2021, 114, 397–402. [Google Scholar] [CrossRef]
- Hickin, M.; Nadel, H.; Schal, C.; Cohen, A.C. Optimization of a Diet for the Greater Wax Moth (Lepidoptera: Pyralidae) Using Full Factorial and Mixture Design. J. Econom. Entomol. 2021, 114, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Champion, O.; Titball, R.; Bates, S. Standardization of G. mellonella Larvae to Provide Reliable and Reproducible Results in the Study of Fungal Pathogens. J. Fungi 2018, 4, 108. [Google Scholar] [CrossRef] [Green Version]
- Andrea, A.; Krogfelt, K.A.; Jenssen, H. Methods and Challenges of Using the Greater Wax Moth (Galleria mellonella) as a Model Organism in Antimicrobial Compound Discovery. Microorganisms 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossoni, R.D.; de Camargo Ribeiro, F.; Santana dos Santos, H.F.; dos Santos, J.D.; de Sousa Oliveira, N.; Theotonio dos Santos Dutra, M.T.; Biazzi de Lapena, S.A.; Junqueira, J.C. Galleria mellonella as an experimental model to study human oral pathogens. Arch. Oral Biol. 2019, 101, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Singkum, P.; Suwanmanee, S.; Pumeesat, P.; Luplertlop, N. A powerful in vivo alternative model in scientific research: Galleria mellonella. Acta Microbiol. Immunol. Hung. 2019, 66, 31–55. [Google Scholar] [CrossRef]
- Büyükgüzel, E.; Tunaz, H.; Stanley, D.; Büyükgüzel, K. Eicosanoids mediate Galleria mellonella cellular immune response to viral infection. J. Insect Physiol. 2007, 53, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Newman, J.F.E.; Porterfield, J.S. The Multiplication of Nodamura Virus in Insect and Mammalian Cell Cultures. J. Gen. Virol. 1975, 26, 15–20. [Google Scholar] [CrossRef]
- Garzon, S.; Charpentier, G.; Kurstak, E. Morphogenesis of the nodamura virus in the larbae of the lepidopteran Galleria mellonella (L.). Arch. Virol. 1978, 56, 61–76. [Google Scholar] [CrossRef]
- Garzon, S.; Strykowski, H.; Charpentier, G. Implication of mitochondria in the replication of Nodamura virus in larvae of the Lepidoptera, Galleria mellonella (L.) and in suckling mice. Arch. Virol. 1990, 113, 165–176. [Google Scholar] [CrossRef]
- 259 Tal, J.; Attathom, T. Insecticidal potential of the insect parvovirusGmDNV. Arch. Insect Biochem. Physiol. 1993, 22, 345–356. [Google Scholar] [CrossRef]
- Meynardier, G.; Vago, C.; Plantevin, G.; Atger, P.X. Virose d’un type habitue1 chez le Lepidoptere, Galleria mellonella. Rev. Zool. Agric. Appl. 1987, 63, 207. [Google Scholar]
- Jafri, R.H.; Khan, A.A. A study of the development of a nuclear-polyhedrosis virus of Heliothis in Galleria mellonella larvae exposed to gamma rays. J. Invertebr. Pathol. 1969, 14, 104. [Google Scholar] [CrossRef]
- Smirnoff, W.A. The amount of potassium and sulfur in healthy and nuclear polyhedrosis virus-infected larvae of Galleria mellonella. J. Invertebr. Pathol. 1971, 18, 295–296. [Google Scholar] [CrossRef]
- Komissarenko, S.V.; Zherebtsova, E.N.; Sutugina, L.P.; Primatchenko, L.V. Non-occluded particles of nuclear polyhedrosis virus infecting Galleria mellonella L.: Titration in vivo and in vitro. Brief report. Arch. Virol. 1979, 61, 347–349. [Google Scholar] [CrossRef]
- Stairs, G.R. Quantitative studies on the infection of Galleria mellonella with a nuclear polyhedrosis virus of Bombyx mori. J. Invertebr. Pathol. 1991, 57, 402–405. [Google Scholar] [CrossRef]
- Demir, İ.; Nalçacioğlu, R.; Mohammad Gholizad, L.; Demirbağ, Z. A highly effective nucleopolyhedrovirus against Malacosoma spp. (Lepidoptera: Lasiocampidae) from Turkey: Isolation, characterization, phylogeny, and virulence. Turk. J. Agric. For. 2014, 38, 462–470. [Google Scholar] [CrossRef]
- El Husseini, M.M. Pathogenicity of nuclear polyhedrosis virus to Galleria mellonella L. (Lepidoptera: Pyralidae) and its control on stored beeswax foundations. Egypt J. Biol. Pest Control 2020, 30, 101. [Google Scholar] [CrossRef]
- Fraser, M.J.; Hink, W.F. The isolation and characterization of the MP and FP plaque variants of Galleria mellonella nuclear polyhedrosis virus. Virology 1982, 117, 366–378. [Google Scholar] [CrossRef]
- Fraser, M.J.; Stairs, G.R. Susceptibility of Trichoplusia ni, Heliothis zea (Noctuidae), and Manduca sexta (Sphingidae) to a nuclear polyhedrosis virus from Galleria mellonella (Pyralidae). J. Invert. Pathol. 1982, 40, 255–259. [Google Scholar] [CrossRef]
- Miloserdova, V.D.; Sukhorada, E.M. In vitro cultivation of testicular cysts of the greater wax moth and multiplication of the associated nuclear polyhedrosis virus. J. Invert. Pathol. 1975, 26, 7–10. [Google Scholar] [CrossRef]
- Bird, F.T. The development of Tipula iridescent virus in the crane fly, Tipula paludosa Meig., and the wax moth, Galleria mellonella L. Can. J. Microbiol. 1961, 7, 827–830. [Google Scholar] [CrossRef]
- Marina, C.F.; Feliciano, J.M.; Valle, J.; Williams, T. Effect of Temperature, pH, Ion Concentration, and Chloroform Treatment on the Stability of Invertebrate Iridescent Virus 6. J. Invertebr. Pathol. 2000, 75, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Constantino, M.; Christian, P.; Marina, C.F.; Williams, T. A comparison of techniques for detecting Invertebrate iridescent virus J. Virol. Methods 2001, 98, 109–118. [Google Scholar] [CrossRef]
- Rud, Y.; Prylutska, S.; Buchatskyy, L.; Prylutskyy, Y.; Ritter, U.; Scharff, P. Photodynamic inactivation of mosquito iridovirus (MIV) by C60 fullerenes. Mater. Sci. Technol. 2011, 42, 2. [Google Scholar] [CrossRef]
- Rud, Y.; Buchatskyy, L.; Prylutskyy, Y.; Marchenko, O.; Senenko, A.; Schütze, C.; Ritter, U. Using C60 fullerenes for photodynamic inactivation of mosquito iridescent viruses. J. Enzym. Inhib. Med. Chem. 2012, 27, 614–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, O.; Maldonado, G.; Williams, T. An epizootic of patent iridescent virus disease in multiple species of blackflies in Chiapas, Mexico. Med. Vet. Entomol. 2000, 14, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Nalcacioglu, R.; Muratoglu, H.; Yesilyurt, A.; van Oers, M.M.; Vlak, J.M.; Demirbag, Z. Enhanced insecticidal activity of Chilo iridescent virus expressing an insect specific neurotoxin. J. Invertebr. Pathol. 2016, 138, 104–111. [Google Scholar] [CrossRef]
- Younghusband, H.B.; Lee, P.E. Virus-cell studies of tipula iridescent virus in Galleria mellonella (L.): I. Electron microscopy of infection and synthesis of tipula iridescent virus in hemocytes. Virology 1969, 38, 247–254. [Google Scholar] [CrossRef]
- Younghusband, H.B.; Lee, P.E. Cytochemistry and autoradiography of Tipula iridescent virus in Galleria mellonella. Virology 1970, 40, 757–760. [Google Scholar] [CrossRef]
- Jafri, R.H.; Chaudhry, M.B. Development of Tipula iridescent virus (TIV) in Galleria mellonella larvae exposed to gamma radiation. J. Invertebr. Pathol. 1971, 18, 46–50. [Google Scholar] [CrossRef]
- Yule, B.G.; Lee, P.E. A cytological and immunological study of Tipula iridescent virus-infected Galleria mellonella larval hemocytes. Virology 1973, 51, 409–423. [Google Scholar] [CrossRef]
- Krell, P.; Lee, P.E. Polypeptides in Tipula iridescent virus (TIV) and in TIV-infected hemocytes of Galleria mellonella (L.) larvae. Virology 1974, 60, 315–326. [Google Scholar] [CrossRef]
- Tanada, Y.; Tanabe, A.M. Resistance of Galleria mellonella (Linnaeus) to the Tipula iridescent virus at high temperatures. J. Invertebr. Pathol. 1965, 7, 184–188. [Google Scholar] [CrossRef]
- Bailey, L.; Ball, B.V.; Woods, R.D. An Iridovirus from Bees. J. Gen. Virol. 1976, 31, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Lea, M.S. A Sericesthis iridescent virus infection of the hemocytes of the waxmoth, Galleria mellonella (Lepidoptera). J. Invertebr. Pathol. 1985, 46, 219–230. [Google Scholar] [CrossRef]
- Lea, M.S. A Sericesthis iridescent virus infection of the hemocytes of the wax moth Galleria mellonella: Effects on total and differential counts and hemocyte ontogeny. J. Invertebr. Pathol. 1986, 48, 42–51. [Google Scholar] [CrossRef]
- Leutenegger, R. Development of an icosahedral virus in hemocytes of Galleria mellonella (L.). Virology 1964, 24, 200–204. [Google Scholar] [CrossRef]
- Rohel, D.Z.; Chadwick, J.; Faulkner, P. Tests with Inactivated Cricket Paralysis Virus as a Possible Immunogen against a Virus Infection of Galleria mellonella Larvae. Intervirology 1980, 14, 61–68. [Google Scholar] [CrossRef]
- Moore, N.F.; Kearns, A.; Pullin, J.S. Characterization of cricket paralysis virus-induced polypeptides in Drosophila cells. J. Virol. 1980, 33, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Traiyasut, P.; Mookhploy, W.; Kimura, K.; Yoshiyama, M.; Khongphinitbunjong, K.; Chantawannakul, P.; Buawangpong, N.; Saraithong, P.; Burgett, M.; Chukeatirote, E. First detection of honey bee viruses in wax moth. Chiang Mai J. Nat. Sci. 2016, 43, 695–698. [Google Scholar]
- Ozkan, S.; Coutts, R.H. Aspergillus fumigatus mycovirus causes mild hypervirulent effect on pathogenicity when tested on Galleria mellonella. Fungal Genet. Biol. 2015, 76, 20–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barwise, A.H.; Walker, I.O. Studies on the DNA of a virus from Galleria mellonella. FEBS Lett. 1970, 6, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Longworth, J.F.; Carey, G.P. A small RNA virus with a divided genome from Heteronychus arator (F.) [Coleoperai Scarabaeidae]. J. Gen. Virol. 1976, 33, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.N.; Ball, L.A. Recovery of Infectious Pariacoto Virus from cDNA Clones and Identification of Susceptible Cell Lines. J. Virol. 2001, 75, 12220–12227. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.N. Virions of Pariacoto virus contain a minor protein translated from the second AUG codon of the capsid protein open reading frame. J. Gen. Virol. 2003, 84, 2847–2852. [Google Scholar] [CrossRef]
- Longworth, J.F.; Archibald, R.D. A virus of black beetle, Heteronychus arator (F.) (Coleoptera: Scarabaeidae). N. Z. J. Zool. 1975, 2, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Yesilyurt, A.; Muratoglu, H.; Demirbag, Z.; Nalcacioglu, R. Chilo iridescent virus encodes two functional metalloproteases. Arch. Virol. 2019, 164, 657–665. [Google Scholar] [CrossRef]
- Maciel-Vergara, G.; Jensen, A.; Eilenberg, J. Cannibalism as a Possible Entry Route for Opportunistic Pathogenic Bacteria to Insect Hosts, Exemplified by Pseudomonas aeruginosa, a Pathogen of the Giant Mealworm Zophoba. Insects 2018, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- Williams, T.; Thompson, I.P. Fatty acid profiles of invertebrate iridescent viruses. Arch. Virol. 1995, 140, 975–981. [Google Scholar] [CrossRef]
- Dicke, M.; Eilenberg, J.; Salles, J.F.; Jensen, A.B.; Lecocq, A.; Pijlman, G.P.; van Loon, J.J.A.; van Oers, M.M. Edible insects unlikely to contribute to transmission of coronavirus SARS-CoV-2. J. Insects Food Feed 2020, 6, 333–339. [Google Scholar] [CrossRef]
- Lery, X.; Fediere, G.; Taha, A.; Salah, M.; Giannotti, J. A new small RNA virus persistently infecting an established cell line of Galleria mellonella, induced by a heterologous infection. J. Invertebr. Pathol. 1997, 69, 7–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Order | Family | Genus | Species | Common Name | Stage Consumed | Insects Potentially Suitable for Use in Food and Feed in the EU * |
|---|---|---|---|---|---|---|
| Coleoptera | Tenebrionidae | Tenebrio | T. molitor (Linnaeus, 1758) | Mealworm | larvae | X ** |
| T. castaneum (Herbst, 1797) | Red flour beetle | larvae | ||||
| Zophobas | Z. morio (Fabricius, 1776) | Super worms | larvae | X | ||
| Alphitobius | A. diaperinus (Panzer, 1797) | Lesser mealworm | larvae | X ** | ||
| Diptera | Muscidae | Musca | M. domestica (Linnaeus, 1758) | Common housefly | larvae | X ** |
| Stratiomyidae | Hermetia | H. illucens (Linnaeus, 1758) | Black soldier fly | larvae | X ** | |
| Lepidoptera | Pyralidae | Piraliini | G. mellonella (Linnaeus, 1758) | Greater wax moth | larvae | X |
| Achroia | A. grisella (Fabricius, 1794) | Lesser wax moth | larvae | X | ||
| Orthoptera | Gryllidae | Acheta | A. domesticus (Linnaeus, 1758) | House cricket | adult | X ** |
| Gryllodes | G. sigillatus (Walker, 1869) | Banded cricket | adult | X ** | ||
| Gryllus | G. assimilis (Fabricius, 1775) | Jamaican field cricket | adult | X ** | ||
| Gryllus | G. bimaculatus (De Geer, 1773) | Two spotted cricket | adult | |||
| Acrididae | Locusta | L. migratora (Linnaeus, 1758) | African migratory locust | adult | X | |
| Schistocerca | S. gregaria (Forskål, 1775) | Desert locust | adult | |||
| Schistocerca | S. americana (Drury, 1770) | American grasshopper | adult | X |
| Coleopteran Species | Virus Family | Virus Genus | Virus Species | Virus Characteristics | Type of Infection | Vector Status | Stage Involved | Symptoms or Mortality | References |
|---|---|---|---|---|---|---|---|---|---|
| Alphitobus diaperinus | Birnaviridae | Avibirnavirus | IBDV | dsRNA | N, E | M | adult | no | [70,73] |
| Coronaviridae | Gammacoronavirus | TCV | dsRNA | E | M | adult | no | [78] | |
| Dicistroviridae | Triatovirus | BQCV | ssRNA | N | M | adult | no | [80] | |
| Dicistroviridae | Aparavirus | IAPV | ssRNA | N | M | adult | no | [80] | |
| Herpesviridae | Iltovirus | ILTV | dsDNA | N | M | adult/larva | no | [80] | |
| Paramyxoviridae | Avulavirus | NDV | ssRNA | E | M | adult | no | [75] | |
| Poxviridae | Avipoxvirus | FWPV | ssRNA | E | M | adult | no | [75] | |
| Reoviridae | Orthoreovirus | AVR | dsRNA | N, E | M | adult/larva | no | [74,76,77] | |
| Reoviridae | Rotavirus | dsRNA | E | M | larva | no | [76] | ||
| Retroviridae | Alpharetrovirus | ALV | ssRNA | N | M | adult | no | [72] | |
| Tenebrio molitor | Iridoviridae | Iridovirus | IIV-6 | dsDNA | E | B | larva | yes | [85] |
| Iridoviridae | Iridovirus | IIV-29 | dsDNA | N, E | B | larva | yes | [86,87] | |
| Parvoviridae | Densovirus | AdDNV | ssDNA | N | M | larva | no | [88] | |
| Parvoviridae | Densovirus | PmergDNV | ssDNA | B | Bi | larva | yes | [89] | |
| Zophobas morio | Parvoviridae | Densovirus | ZbDNV | ssDNA | N, E | B | larva | yes | [100] |
| Parvoviridae | Densovirus | ssDNA | N | nd | larva | yes | [99] |
| Orthopteran Species | Virus Family | Virus Genus | Virus Species | Virus Characteristics | Type of Infection | Vector Status | Stage Involved | Symptoms or Mortality | References |
|---|---|---|---|---|---|---|---|---|---|
| Acheta domesticus | Baculoviridae | Alphabaculovirus | AcMNPV, AcMNPV.AaIT, AcJHE.SG AcMNPV | dsDNA | E | M | adult | no | [119,120] |
| Dicistroviridae | Cripavirus | BCV | ssRNA | N | M | adult | no | [121] | |
| Dicistroviridae | Cripavirus | CrPV | ssRNA | N | B | nd | yes | [117,143] | |
| Iflaviridae | Iflavirus | AdIV | ssRNA | N | B | adult/nymph | no | [115] | |
| Iflaviridae | Iflavirus | Chequa iflavirus | ssRNA | E | Bi | nd | no | [124] | |
| Iridoviridae | Iridovirus | CrIV | dsDNA | N, E | B | adult/nymph | yes | [58,116] | |
| Iridoviridae | Iridovirus | IIV-6 | dsDNA | E | B | nymph | yes | [107] | |
| nd | nd | AdVVV | ssDNA | N | nd | nd | nd | [107,112,113] | |
| Parvoviridae | nd | AdMADV | ssDNA | N | nd | nd | nd | [114] | |
| Parvoviridae | Densovirus | AdDNV | ssDNA | N, E | B | adult/nymph | yes | [44,88,107,108,109,110,158] | |
| Parvoviridae | Densovirus | PmergDNV | ssDNA | E | Bi | nd | yes | [89,123] | |
| Phenuiviridae | nd | Bunya-like virus | ssRNA | E | Bi | nd | no | [124] | |
| Solinviviridae | Invictavirus | SINV-3 | ssRNA | E | M | adult/nymph | no | [122] | |
| Nudiviridae | Alphanudivirus | GbNV | dsDNA | N | B | adult/nymph | yes | [107] | |
| Gryllodes sigillatus | Parvoviridae | Densovirus | AdDNV | ssDNA | E | B | nd | nd | [44] |
| Gryllus assimilis | Iridoviridae | Iridovirus | CrIV | dsDNA | B | nymph | nd | [58] | |
| nd | nd | AdVVV | ssDNA | N | nd | nd | nd | [113] | |
| Parvoviridae | Densovirus | AdDNV | ssDNA | N, E | resistant | [44,88] | |||
| Gryllus bimaculatus | Dicistroviridae | Cripavirus | CrPV | ssRNA | N | B | nymph | yes | [117,118] |
| Iridoviridae | Iridovirus | GbIV | dsDNA | N | B | adult | yes | [57,138] | |
| Iridoviridae | Iridovirus | CrIV | dsDNA | N, E | B | adult/nymph | yes | [58,116] | |
| Iridoviridae | Iridovirus | Liz_CrIV | dsDNA | E | B | nymph | yes | [60] | |
| Iridoviridae | Iridovirus | Cham_IIV | dsDNA | E | B | nymph | yes | [45] | |
| Iridoviridae | Iridovirus | IIV-6 | dsDNA | N, E | B | nymph | yes | [58] | |
| Nudiviridae | Alphanudivirus | GbNV | dsDNA | N, E | B | adult/nymph | yes | [140,141] | |
| Parvoviridae | Densovirus | AdDNV | ssDNA | N, E | resistant | [44,90] | |||
| Locusta migratoria | Baculoviridae | Alphabaculovirus | SINV | dsDNA | E | B | nymph | yes | [148,149] |
| Iridoviridae | iridovirus | CrIV | dsDNA | E | B | adult/nymph | yes | [116] | |
| Iridoviridae | iridovirus | IIV-6 | dsDNA | E | B | nymph | yes | [58] | |
| Poxviridae | Betaentomopoxvirus | MsEPV | dsDNA | E | B | nymph | yes | [145,146] | |
| Poxviridae | Betaentomopoxvirus | dsDNA | N | B | nd | yes | [144] | ||
| Reoviridae | Cypovirus | CPV | dsDNA | E | B | adult/nymph | [150] | ||
| Schistocerca americana | Picornaviridae | nd | CAV | ssRNA | N, E | B | nymph | yes | [156,157] |
| Poxviridae | Betaentomopoxvirus | MsEPV | dsDNA | N | B | nymph | yes | [154,155] | |
| Schistocerca gregaria | Iridoviridae | Iridovirus | IIV-6 | dsDNA | E | B | nymph | yes | [58] |
| Iridoviridae | Iridovirus | CrIV | dsDNA | E | B | nymph | yes | [58] | |
| nd | nd | Schistocerca virus | nd | N | nd | adult | nd | [151] | |
| Poxviridae | Betaentomopoxvirus | dsDNA | N | nd | nd | nd | [152] |
| Dipteran Species | Virus Family | Virus Genus | Virus Species | Virus Characteristics | Type of Infection | Vector Status | Stage Involved | Symptoms or Mortality | References |
|---|---|---|---|---|---|---|---|---|---|
| Hermetia illucens | none | ||||||||
| Musca domestica | Arteriviridae | Betaarterivirus | PRRSV | ssRNA | N/E | M | adult | no | [224,225,226,227,228] |
| Asfarviridae | Asfivirus | ASFV | dsDNA | N | M | adult | no | [231,232] | |
| Circoviridae | Circovirus | PCV2 | ssDNA | N/E | M | adult | no | [229] | |
| Coronaviridae | Alphacoronavirus | PEDV | ssRNA | E | M | adult | no | [230] | |
| Coronaviridae | Gammacoronavirus | TCV | ssRNA | E | M | adult | no | [213] | |
| Filoviridae | Ebolavirus | EBOV | ssRNA | E | M | adult | no | [198] | |
| Herpesviridae | Varicellovirus | PRV-1 | ssDNA | E | M | adult | no | [233] | |
| Hytrosaviridae | Muscavirus | MdSGHV | dsDNA | N/E | B | adult | yes | [199,210,240] | |
| Reoviridae | nd | Idno-3 | dsRNA | N/E | B | adult/larva | yes | [188,211] | |
| Orthomyxovirdae | Alphainfluenzavirus | HPAIV H5N1; LPAI H9N2 | ssRNA | N/E | M | adult | no | [212,213,214,215,216] | |
| Paramyxoviridae | Avulavirus | NDV; ENDV | ssRNA | N/E | M | adult/larva | no | [218,219,220,221,222] | |
| Parvoviridae | Amdoparvovirus | AMDV | ssDNA | N | M | adult | no | [237] | |
| Parvoviridae | Protoparvovirus | MEV | ssDNA | E | M | adult | no | [236] | |
| Phenuiviridae | Phlebovirus | RVFV | dsRNA | E | M | adult | no | [235] | |
| Picornaviridae | Enterovirus | Ento C | ssRNA | E | M | adult/larva | yes | [191,192,193,194,195] | |
| Picornaviridae | Enterovirus | Coxs B | ssRNA | N | M | adult | no | [196] | |
| Picornaviridae | Enterovirus | Coxs C | ssRNA | E | M | adult | no | [195] | |
| Picornaviridae | Senecavirus | SVA | ssRNA | N | M | adult | no | [234] | |
| Poxviridae | Capripoxvirus | LSDV | dsDNA | N | M | adult | no | [238] | |
| Reoviridae | Rotavirus | SA11 | dsRNA | E | M | adult | no | [197] | |
| Retroviridae | Gammaretrovirus | REV | ssRNA | N/E | M | adult | no | [223] |
| Lepidopteran Species | Virus Family | Virus Genus | Virus Species | Virus Characteristics | Type of Infection | Vector Status | Stage Involved | Symptoms or Mortality | References |
|---|---|---|---|---|---|---|---|---|---|
| Achroia grisella | None | ||||||||
| Galleria mellonella | Baculoviridae | Alphabaculovirus | NPVs | dsDNA | E | B | larva | yes | [261,262] |
| Baculoviridae | Alphabaculovirus | BmNPV | dsDNA | E | B | larva | yes | [263] | |
| Baculoviridae | Alphabaculovirus | GmMNPV | dsDNA | N, E | B | larva | yes | [265,266,267,268] | |
| Baculoviridae | Alphabaculovirus | HNPV | dsDNA | E | B | larva | yes | [260] | |
| Baculoviridae | Alphabaculovirus | ManeNPV-T3 | dsDNA | E | B | larva | yes | [264] | |
| Baculoviridae | Alphabaculovirus | AcMNPV CIV-MMPs | dsDNA | E | B | larva | yes | [295] | |
| Dicistroviridae | Aparavirus | IAPV | ssRNA | N | nd | larva | nd | [288] | |
| Dicistroviridae | Triatovirus | BQCV | ssRNA | N | nd | larva | nd | [288] | |
| Dicistroviridae | Cripavirus | CrPV | ssRNA | E | B | larva | yes | [286,287] | |
| Herpesviridae | Simplexvirus | BHSV-1 | dsDNA | E | Bi | larva | no | [254] | |
| Iridoviridae | Iridovirus | IIV-6 | dsDNA | E | B | larva | yes | [61,270,271,274,275] | |
| Iridoviridae | Iridovirus | AgIIV | dsDNA | E | B | larva | yes | [270] | |
| Iridoviridae | Iridovirus | IIVs | dsDNA | E | B | larva | yes | [50] | |
| Iridoviridae | Iridovirus | SIV | dsDNA | E | B | larva | yes | [283,284,285] | |
| Iridoviridae | Iridovirus | TIV | dsDNA | E | B | larva | yes | [269,276,277,278,279,280,281] | |
| Iridoviridae | Iridovirus | CrIV | dsDNA | E | B | larva | yes | [61,116] | |
| Iridoviridae | Iridovirus | AIV | dsDNA | E | resistant | larva | [282] | ||
| Iridoviridae | Iridovirus | MIV | dsDNA | E | B | larva | yes | [272,274] | |
| Iridoviridae | Iridovirus | IIV-29 | dsDNA | E | B | larva | no | [88] | |
| Iridoviridae | Iridovirus | IIV-6 recombinant | dsDNA | E | B | larva | yes | [275] | |
| nd | nd | GFV | dsDNA | N | nd | larva | nd | [290] | |
| nd | nd | HaV | small RNA | E | B | larva | yes | [291,294] | |
| nd | nd | Mycovirus A78 | nd | N, E | nd | larva | yes | [289] | |
| Nodaviridae | Alphanodavirus | NoV | ssRNA | E | larva | yes | [255,256,257] | ||
| Nodaviridae | Alphanodavirus | PaV | ssRNA | E | Bi | larva | yes | [292,293] | |
| Parvoviridae | Densovirus | AdDNV | ssDNA | E | resistant | larva | no | [90] | |
| Parvoviridae | Densovirus | GmDNV | ssDNA | E | B | larva | yes | [258] | |
| Picornaviridae | nd | GmclV | RNA | E | B | larva | yes | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertola, M.; Mutinelli, F. A Systematic Review on Viruses in Mass-Reared Edible Insect Species. Viruses 2021, 13, 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/v13112280
Bertola M, Mutinelli F. A Systematic Review on Viruses in Mass-Reared Edible Insect Species. Viruses. 2021; 13(11):2280. https://0-doi-org.brum.beds.ac.uk/10.3390/v13112280
Chicago/Turabian StyleBertola, Michela, and Franco Mutinelli. 2021. "A Systematic Review on Viruses in Mass-Reared Edible Insect Species" Viruses 13, no. 11: 2280. https://0-doi-org.brum.beds.ac.uk/10.3390/v13112280