Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Whole-Cell Inactivated N. gonorrhoeae Vaccine Antigen
2.2.2. Gonococci Vaccine Microparticles and Adjuvant Microparticles Formulation
2.2.3. Autophagy Induction Assessment
2.2.4. Nitric Oxide Release Measurement by the Griess Reaction
2.2.5. In Vitro Cytotoxicity Evaluation Using the MTT Method
2.2.6. Dendritic Cell Uptake Study
2.2.7. Quantification of MHCI, MHCII, CD40 and CD86 Surface Expression on Dendritic Cells
2.2.8. Evaluation of Surface Expression of Death Receptor CD95 on Dendritic Cells
2.2.9. Statistical Analysis
3. Results
3.1. Characterization of the Microparticles
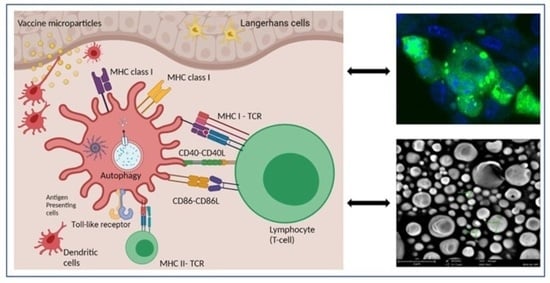
3.2. Adjuvanted Gonococci Vaccine Microparticles Enhanced Autophagy Induction in Antigen Presenting Cells
3.3. Antigen Presentation Assessment in Antigen Presenting Cells Exposed to Gonococci Vaccine Microparticles with or without Adjuvant MP
3.3.1. Adjuvanted Gonococci Vaccine Microparticles Enhanced Nitric Oxide Release from Induced Macrophages
3.3.2. Cytotoxicity Evaluation of Gonococci Vaccine MP
3.3.3. Dendritic Cell Uptake of Microparticles
3.3.4. Expression of Antigen Presenting and Co-Stimulatory Molecules on the Surface of Dendritic Cells
3.4. Expression of CD95 Death Receptor on the Surface of Antigen Presenting Cells Induced with Gonococci Vaccine Microparticles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sexually Transmitted Infections (STIs). Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 15 December 2021).
- CDC. STI Prevalence, Incidence, and Cost Estimates Infographic. Available online: https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm (accessed on 18 May 2021).
- Golparian, D.; Shafer, W.M.; Ohnishi, M.; Unemo, M. Importance of Multidrug Efflux Pumps in the Antimicrobial Resistance Property of Clinical Multidrug-Resistant Isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2014, 58, 3556–3559. [Google Scholar] [CrossRef] [Green Version]
- Kirkcaldy, R.D.; Weston, E.; Segurado, A.C.; Hughes, G. Epidemiology of Gonorrhoea: A Global Perspective. Sex. Health 2019, 16, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Detailed STD Facts—Gonorrhea. Available online: https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm (accessed on 16 August 2021).
- Antibiotic Resistant Gonorrhea—STD Information from CDC. Available online: https://www.cdc.gov/std/gonorrhea/arg/default.htm (accessed on 12 August 2021).
- Edwards, J.L.; Jennings, M.P.; Apicella, M.A.; Seib, K.L. Is Gonococcal Disease Preventable? The Importance of Understanding Immunity and Pathogenesis in Vaccine Development. Crit. Rev. Microbiol. 2016, 42, 928–941. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, S.L.; Ndowa, F.; Hook, E.W.; Deal, C.; Bachmann, L.; Abu-Raddad, L.; Chen, X.-S.; Jerse, A.; Low, N.; MacLennan, C.A.; et al. Gonococcal Vaccines: Public Health Value and Preferred Product Characteristics; Report of a WHO Global Stakeholder Consultation, January 2019. Vaccine 2020, 38, 4362–4373. [Google Scholar] [CrossRef] [PubMed]
- Jerse, A.E.; Bash, M.C.; Russell, M.W. Vaccines against Gonorrhea: Current Status and Future Challenges. Vaccine 2014, 32, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemo, M.; del Rio, C.; Shafer, W.M. Antimicrobial Resistance Expressed by Neisseria gonorrhoeae: A Major Global Public Health Problem in the 21st Century. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzler, L.M.; Feavers, I.M.; Gray-Owen, S.D.; Jerse, A.E.; Rice, P.A.; Deal, C.D. Summary and Recommendations from the National Institute of Allergy and Infectious Diseases (NIAID) Workshop “Gonorrhea Vaccines: The Way Forward”. Clin. Vaccine Immunol. CVI 2016, 23, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, L.; Diena, B.B.; Ashton, F.A.; Wallace, R.; Kenny, C.P.; Znamirowski, R.; Ferrari, H.; Atkinson, J. Gonococcal Vaccine Studies in Inuvik. Can. J. Public Health Rev. Can. Sante Publique 1974, 65, 29–33. [Google Scholar]
- Arko, R.J.; Duncan, W.P.; Brown, W.J.; Peacock, W.L.; Tomizawa, T. Immunity in Infection with Neisseria gonorrhoeae: Duration and Serological Response in the Chimpanzee. J. Infect. Dis. 1976, 133, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Thomas, C.E.; Chen, C.-J.; Van Dam, C.N.; Johnston, R.E.; Davis, N.L.; Sparling, P.F. Comparison of Immune Responses to Gonococcal PorB Delivered as Outer Membrane Vesicles, Recombinant Protein, or Venezuelan Equine Encephalitis Virus Replicon Particles. Infect. Immun. 2005, 73, 7558–7568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetzler, L.M.; Blake, M.S.; Barry, K.; Gotschlich, E.C. Gonococcal Porin Vaccine Evaluation: Comparison of Por Proteosomes, Liposomes, and Blebs Isolated from Rmp Deletion Mutants. J. Infect. Dis. 1992, 166, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, C.R.; Nassif, X. Analysis of the Genetic Differences between Neisseria Meningitidis and Neisseria gonorrhoeae: Two Closely Related Bacteria Expressing Two Different Pathogenicities. Proc. Natl. Acad. Sci. USA 1996, 93, 11109–11114. [Google Scholar] [CrossRef] [Green Version]
- Marjuki, H.; Topaz, N.; Joseph, S.J.; Gernert, K.M.; Kersh, E.N.; Antimicrobial-Resistant Neisseria gonorrhoeae Working Group; Wang, X. Genetic similarity of gonococcal homologs to meningococcal outer membrane proteins of serogroup B vaccine. Mbio 2019, 10, e01668-19. [Google Scholar]
- Bagwe, P.; Bajaj, L.; Gala, R.; Zughaier, S.M.; Uddin, M.N.; D’Souza, M.J. Meningococcal Vaccines: Challenges and Prospects. Vaccines 2020, 8, 738. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Tan, A.; Borrow, R.; Seib, K.L. The Serogroup B Meningococcal Vaccine Bexsero Elicits Antibodies to Neisseria gonorrhoeae. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019, 69, 1101–1111. [Google Scholar] [CrossRef]
- Abara, W.E.; Bernstein, K.T.; Lewis, F.M.T.; Schillinger, J.A.; Feemster, K.; Pathela, P.; Hariri, S.; Islam, A.; Eberhart, M.; Cheng, I.; et al. Effectiveness of a Serogroup B Outer Membrane Vesicle Meningococcal Vaccine against Gonorrhoea: A Retrospective Observational Study. Lancet Infect. Dis. 2022; in press. [Google Scholar] [CrossRef]
- Kirby Institute. A Multi-Centre Randomised Controlled Trial Evaluating the Efficacy of the Four-Component Meningococcal B Vaccine, 4CMenB (Bexsero®), in the Prevention of Neisseria gonorrhoeae Infection in Gay and Bisexual Men. Clinical Trial Registration NCT04415424. Available online: https://clinicaltrials.gov/ct2/show/NCT04415424 (accessed on 6 June 2021).
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase II Randomized, Observer-Blind, Placebo-Controlled Study, to Assess the Efficacy of Meningococcal Group B Vaccine RMenB+OMV NZ (Bexsero) in Preventing Gonococcal Infection. Clinical Trial Registration NCT04350138. Available online: https://clinicaltrials.gov/ct2/show/NCT04350138 (accessed on 6 June 2021).
- University of North Carolina, Chapel Hill. Institute for Global Health and Infectious Diseases (IGHID) 11911—Cross-Reactive N. Gonorrhoeae Immune Responses Induced by a N. Meningitidis Vaccine. Clinical Trial Registration NCT04094883. Available online: https://clinicaltrials.gov/ct2/show/NCT04094883 (accessed on 16 June 2021).
- National Institute of Allergy and Infectious Diseases (NIAID). Mucosal Immune Responses Against Neisseria gonorrhoeae Following Meningococcal Immunization in Healthy Young Adults. Clinical Trial Registration NCT04722003. Available online: https://clinicaltrials.gov/ct2/show/NCT04722003 (accessed on 16 June 2021).
- University of Oxford. Use of Bexsero Immunisation to Detect Cross Reactive Antigens and Anti-Gonococcal Antibodies in Key Populations in Kenya. Clinical Trial Registration NCT04297436. Available online: https://clinicaltrials.gov/ct2/show/NCT04297436 (accessed on 4 June 2021).
- ScienceDirect Topics. Antigen Presentation—An overview. Available online: https://0-www-sciencedirect-com.brum.beds.ac.uk/topics/medicine-and-dentistry/antigen-presentation (accessed on 14 March 2021).
- Wieczorek, M.; Abualrous, E.T.; Sticht, J.; Álvaro-Benito, M.; Stolzenberg, S.; Noé, F.; Freund, C. Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Front. Immunol. 2017, 8, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JNCI: Journal of the National Cancer Institute, Oxford Academic. MHC Class I Antigen Processing and Presenting Machinery: Organization, Function, and Defects in Tumor Cells. Available online: https://0-academic-oup-com.brum.beds.ac.uk/jnci/article/105/16/1172/942776 (accessed on 16 August 2021).
- The MHC Class I Antigen Presentation Pathway: Strategies for Viral Immune Evasion. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC1783040/ (accessed on 16 August 2021).
- Gala, R.P.; Zaman, R.U.; D’Souza, M.J.; Zughaier, S.M. Novel Whole-Cell Inactivated Neisseria gonorrhoeae Microparticles as Vaccine Formulation in Microneedle-Based Transdermal Immunization. Vaccines 2018, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Springer. Induction of Death Receptor CD95 and Co-stimulatory Molecules CD80 and CD86 by Meningococcal Capsular Polysaccharide-Loaded Vaccine Nanoparticles. Available online: https://0-link-springer-com.brum.beds.ac.uk/article/10.1208/s12248-014-9635-2 (accessed on 18 April 2021).
- Ubale, R.V.; D’Souza, M.J.; Infield, D.T.; McCarty, N.A.; Zughaier, S.M. Formulation of Meningococcal Capsular Polysaccharide Vaccine-Loaded Microparticles with Robust Innate Immune Recognition. J. Microencapsul. 2013, 30, 28–41. [Google Scholar] [CrossRef] [PubMed]
- AddaVaxTM. Available online: https://www.invivogen.com/addavax (accessed on 18 August 2021).
- CDC. Adjuvanted Flu Vaccine. Available online: https://www.cdc.gov/flu/prevent/adjuvant.htm (accessed on 16 December 2021).
- Gala, R.P.; D’Souza, M.; Zughaier, S.M. Evaluation of Various Adjuvant Nanoparticulate Formulations for Meningococcal Capsular Polysaccharide-Based Vaccine. Vaccine 2016, 34, 3260–3267. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’Souza, M.J.; Zughaier, S.M. Microneedles: A New Generation Vaccine Delivery System. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Bejugam, N.K.; Gayakwad, S.G.; Uddin, A.N.; D’Souza, M.J. Microencapsulation of Protein into Biodegradable Matrix: A Smart Solution Cross-Linking Technique. J. Microencapsul. 2013, 30, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Annual Review of Pathology: Mechanisms of Disease. Autophagy: Basic Principles and Relevance to Disease. Available online: https://0-www-annualreviews-org.brum.beds.ac.uk/doi/abs/10.1146/annurev.pathmechdis.2.010506.091842 (accessed on 19 August 2021).
- Zughaier, S.M.; Kandler, J.L.; Balthazar, J.T.; Shafer, W.M. Phosphoethanolamine Modification of Neisseria gonorrhoeae Lipid A Reduces Autophagy Flux in Macrophages. PLoS ONE 2015, 10, e0144347. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Shafer, W.M.; Stephens, D.S. Antimicrobial Peptides and Endotoxin Inhibit Cytokine and Nitric Oxide Release but Amplify Respiratory Burst Response in Human and Murine Macrophages. Cell. Microbiol. 2005, 7, 1251–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Galluzzi, L.; Zitvogel, L.; Kroemer, G. Autophagy and Cellular Immune Responses. Immunity 2013, 39, 211–227. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Deretic, V. Unveiling the Roles of Autophagy in Innate and Adaptive Immunity. Nat. Rev. Immunol. 2007, 7, 767–777. [Google Scholar] [CrossRef]
- Shafer, W.M.; Onunka, V.C.; Jannoun, M.; Huthwaite, L.W. Molecular Mechanism for the Antigonococcal Action of Lysosomal Cathepsin G. Mol. Microbiol. 1990, 4, 1269–1277. [Google Scholar] [CrossRef]
- Leleux, J.; Roy, K. Micro and Nanoparticle-Based Delivery Systems for Vaccine Immunotherapy: An Immunological and Materials Perspective. Adv. Healthc. Mater. 2013, 2, 72–94. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Silva, A.L.; Soema, P.C.; Slütter, B.; Ossendorp, F.; Jiskoot, W. PLGA Particulate Delivery Systems for Subunit Vaccines: Linking Particle Properties to Immunogenicity. Hum. Vaccines Immunother. 2016, 12, 1056–1069. [Google Scholar] [CrossRef]
- Kanojia, G.; ten Have, R.; Soema, P.C.; Frijlink, H.; Amorij, J.-P.; Kersten, G. Developments in the Formulation and Delivery of Spray Dried Vaccines. Hum. Vaccines Immunother. 2017, 13, 2364–2378. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-Based Drug Delivery: Harnessing Nature to Cure Disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Schijns, V.E.J.C.; Lavelle, E.C. Trends in Vaccine Adjuvants. Expert Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef]
- Ramanathan, V.D.; Badenoch-Jones, P.; Turk, J.L. Complement Activation by Aluminium and Zirconium Compounds. Immunology 1979, 37, 881–888. [Google Scholar]
- Seubert, A.; Calabro, S.; Santini, L.; Galli, B.; Genovese, A.; Valentini, S.; Aprea, S.; Colaprico, A.; D’Oro, U.; Giuliani, M.M.; et al. Adjuvanticity of the Oil-in-Water Emulsion MF59 Is Independent of Nlrp3 Inflammasome but Requires the Adaptor Protein MyD88. Proc. Natl. Acad. Sci. USA 2011, 108, 11169–11174. [Google Scholar] [CrossRef] [Green Version]
- Rimaniol, A.-C.; Gras, G.; Verdier, F.; Capel, F.; Grigoriev, V.B.; Porcheray, F.; Sauzeat, E.; Fournier, J.-G.; Clayette, P.; Siegrist, C.-A.; et al. Aluminum Hydroxide Adjuvant Induces Macrophage Differentiation towards a Specialized Antigen-Presenting Cell Type. Vaccine 2004, 22, 3127–3135. [Google Scholar] [CrossRef]
- Infection and Immunity. Investigation of Role of Nitric Oxide in Protection from Bordetella pertussis Respiratory Challenge. Available online: https://iai.asm.org/content/70/2/679 (accessed on 18 April 2021).
- Ghislat, G.; Lawrence, T. Autophagy in Dendritic Cells. Cell. Mol. Immunol. 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Ireland, J.M.; Unanue, E.R. Autophagy in Antigen-Presenting Cells Results in Presentation of Citrullinated Peptides to CD4 T Cells. J. Exp. Med. 2011, 208, 2625–2632. [Google Scholar] [CrossRef]
- Wu, H.; Che, X.; Zheng, Q.; Wu, A.; Pan, K.; Shao, A.; Wu, Q.; Zhang, J.; Hong, Y. Caspases: A Molecular Switch Node in the Crosstalk between Autophagy and Apoptosis. Int. J. Biol. Sci. 2014, 10, 1072–1083. [Google Scholar] [CrossRef]
- Han, J.; Hou, W.; Goldstein, L.A.; Stolz, D.B.; Watkins, S.C.; Rabinowich, H. A Complex between Atg7 and Caspase-9: A Novel Mechanism of Cross-Regulation between Autophagy and Apoptosis. J. Biol. Chem. 2014, 289, 6485–6497. [Google Scholar] [CrossRef] [Green Version]
- Monari, C.; Paganelli, F.; Bistoni, F.; Kozel, T.R.; Vecchiarelli, A. Capsular Polysaccharide Induction of Apoptosis by Intrinsic and Extrinsic Mechanisms. Cell. Microbiol. 2008, 10, 2129–2137. [Google Scholar] [CrossRef]
- Bossaller, L.; Chiang, P.-I.; Schmidt-Lauber, C.; Ganesan, S.; Kaiser, W.J.; Rathinam, V.A.K.; Mocarski, E.S.; Subramanian, D.; Green, D.R.; Silverman, N.; et al. Cutting Edge: FAS (CD95) Mediates Noncanonical IL-1β and IL-18 Maturation via Caspase-8 in an RIP3-Independent Manner. J. Immunol. 2012, 189, 5508–5512. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, Y.; Oshima, S.; Nibe, Y.; Kobayashi, M.; Maeyashiki, C.; Nemoto, Y.; Nagaishi, T.; Okamoto, R.; Tsuchiya, K.; Nakamura, T.; et al. RIPK3 Regulates P62–LC3 Complex Formation via the Caspase-8-Dependent Cleavage of P62. Biochem. Biophys. Res. Commun. 2015, 456, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Oral, O.; Oz-Arslan, D.; Itah, Z.; Naghavi, A.; Deveci, R.; Karacali, S.; Gozuacik, D. Cleavage of Atg3 Protein by Caspase-8 Regulates Autophagy during Receptor-Activated Cell Death. Apoptosis Int. J. Program. Cell Death 2012, 17, 810–820. [Google Scholar] [CrossRef]
- Pericolini, E.; Cenci, E.; Monari, C.; De Jesus, M.; Bistoni, F.; Casadevall, A.; Vecchiarelli, A. Cryptococcus Neoformans Capsular Polysaccharide Component Galactoxylomannan Induces Apoptosis of Human T-Cells through Activation of Caspase-8. Cell. Microbiol. 2006, 8, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Villena, S.N.; Pinheiro, R.O.; Pinheiro, C.S.; Nunes, M.P.; Takiya, C.M.; DosReis, G.A.; Previato, J.O.; Mendonça-Previato, L.; Freire-de-Lima, C.G. Capsular Polysaccharides Galactoxylomannan and Glucuronoxylomannan from Cryptococcus Neoformans Induce Macrophage Apoptosis Mediated by Fas Ligand. Cell. Microbiol. 2008, 10, 1274–1285. [Google Scholar] [CrossRef]
- The Journal of Immunology. Trypanosoma Cruzi Infection Selectively Renders Parasite-Specific IgG+ B Lymphocytes Susceptible to Fas/Fas Ligand-Mediated Fratricide. Available online: https://www.jimmunol.org/content/168/8/3965.short (accessed on 26 August 2021).
- Pathogen-Induced Proapoptotic Phenotype and High CD95 (Fas) Expression Accompany a Suboptimal CD8+ T-Cell Response: Reversal by Adenoviral Vaccine. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC3355083/ (accessed on 26 August 2021).







| Gc-MP | Alum MP | AddaVax MP | |
|---|---|---|---|
| Protein content in fixed Gc | 2.5 mg/mL | - | - |
| Percent yield | 85% w/w | 88% w/w | 84% w/w |
| Particle size | 3.5 ± 1.2 μm | 4.3 ± 0.7 μm | 2.18 ± 0.9 μm |
| Polydispersity index (PDI) | 0.34 | 0.60 | 0.558 |
| Zeta potential | −25 ± 5.79 mV | −22 ± 2.33 mV | −16.95 ± 1.53 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagwe, P.; Bajaj, L.; Gala, R.P.; D‘Souza, M.J.; Zughaier, S.M. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation. Vaccines 2022, 10, 983. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines10070983
Bagwe P, Bajaj L, Gala RP, D‘Souza MJ, Zughaier SM. Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation. Vaccines. 2022; 10(7):983. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines10070983
Chicago/Turabian StyleBagwe, Priyal, Lotika Bajaj, Rikhav P. Gala, Martin J. D‘Souza, and Susu M. Zughaier. 2022. "Assessment of In Vitro Immunostimulatory Activity of an Adjuvanted Whole-Cell Inactivated Neisseria gonorrhoeae Microparticle Vaccine Formulation" Vaccines 10, no. 7: 983. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines10070983