Interferon-α-Induced Dendritic Cells Generated with Human Platelet Lysate Exhibit Elevated Antigen Presenting Ability to Cytotoxic T Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Cell Separation and Culture
2.2.1. Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
2.2.2. Generation of IFN-DCs
2.3. Cellular Morphology Observations
2.4. Cell Surface Marker Analysis
2.5. Analysis of Endocytic and Proteolytic Activities
2.6. CTL Induction In Vitro
2.7. Detection of Cytokine Production
2.8. Enzyme-Linked Immunosorbent Spot (ELISpot) Assay
2.9. Statistical Analysis
3. Results
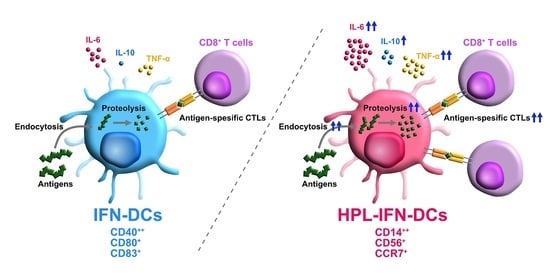
3.1. Generation of IFN-DCs Using Serum-Free Medium Supplemented with HPL
3.2. Phenotypic Comparison between IFN-DCs and HPL-IFN-DCs
3.3. HPL-IFN-DCs Are Highly Capable of Inducing Antigen-Specific CTLs
3.4. CTLs Induced by IFN-DCs or HPL-IFN-DCs Produced IFN-γ in Response to MART-1 Peptides
3.5. HPL Upregulated the Endocytic and Proteolytic Activities of IFN-DCs
3.6. Compared with IFN-DCs, HPL-IFN-DCs Showed Higher IL-6, IL-10, and TNF-α Production Levels
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banchereau, J.; Steinman, R.M. Dendritic Cells and the Control of Immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Granot, T.; Senda, T.; Carpenter, D.J.; Matsuoka, N.; Weiner, J.; Gordon, C.L.; Miron, M.; Kumar, B.V.; Griesemer, A.; Ho, S.H.; et al. Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 2017, 46, 504–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collin, M.; McGovern, N.; Haniffa, M. Human Dendritic Cell Subsets. Immunology 2013, 140, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-Cell RNA-Seq Reveals New Types of Human Blood Dendritic Cells, Monocytes, and Progenitors. Science 2017, 356. [Google Scholar] [CrossRef] [Green Version]
- McGovern, N.; Schlitzer, A.; Gunawan, M.; Jardine, L.; Shin, A.; Poyner, E.; Green, K.; Dickinson, R.; Wang, X.N.; Low, D.; et al. Human Dermal CD14+ Cells Are a Transient Population of Monocyte-Derived Macrophages. Immunity 2014, 41, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Wylie, B.; Macri, C.; Mintern, J.D.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers 2019, 11, 521. [Google Scholar] [CrossRef] [Green Version]
- Akagawa, K.S.; Takasuka, N.; Nozaki, Y.; Komuro, I.; Azuma, M.; Ueda, M.; Naito, M.; Takahashi, K. Generation of CD1+RelB+ Dendritic Cells and Tartrate-Resistant Acid Phosphatase-Positive Osteoclast-Like Multinucleated Giant Cells from Human Monocytes. Blood 1996, 88, 4029–4039. [Google Scholar] [CrossRef] [Green Version]
- Fu, C.; Jiang, A. Dendritic Cells and CD8 T Cell Immunity in Tumor Microenvironment. Front. Immunol. 2018, 9, 3059. [Google Scholar] [CrossRef] [Green Version]
- Koya, T.; Date, I.; Kawaguchi, H.; Watanabe, A.; Sakamoto, T.; Togi, M.; Kato, T.; Yoshida, K.; Kojima, S.; Yanagisawa, R.; et al. Dendritic Cells Pre-Pulsed with Wilms’ Tumor 1 in Optimized Culture for Cancer Vaccination. Pharmaceutics 2020, 12, 305. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, R.; Koizumi, T.; Koya, T.; Sano, K.; Koido, S.; Nagai, K.; Kobayashi, M.; Okamoto, M.; Sugiyama, H.; Shimodaira, S. WT1-Pulsed Dendritic Cell Vaccine Combined with Chemotherapy for Resected Pancreatic Cancer in a Phase I Study. Anticancer Res. 2018, 38, 2217–2225. [Google Scholar] [CrossRef] [Green Version]
- Pierret, L.; Wilgenhof, S.; Corthals, J.; Roelandt, T.; Thielemans, K.; Neyns, B. Correlation between Prior Therapeutic Dendritic Cell Vaccination and the Outcome of Patients with Metastatic Melanoma Treated with Ipilimumab. J. Clin. Oncol. 2009, 27, e20006. [Google Scholar] [CrossRef]
- Nesselhut, J.; Marx, D.; Lange, H.; Regalo, G.; Cillien, N.; Chang, R.Y.; Nesselhut, T. Systemic Treatment with Anti-PD-1 Antibody Nivolumab in Combination with Vaccine Therapy in Advanced Pancreatic Cancer. J. Clin. Oncol. 2016, 34, 3092. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized Cancer Vaccine Effectively Mobilizes Antitumor T Cell Immunity in Ovarian Cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.Y.; Gu, C.; Min, J.; Chu, Z.H.; Ou, Q.J. Maturation Induction of Human Peripheral Blood Mononuclear Cell-Derived Dendritic Cells. Exp. Ther. Med. 2012, 4, 131–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimodaira, S.; Yanagisawa, R.; Koya, T.; Hirabayashi, K.; Higuchi, Y.; Sakamoto, T.; Togi, M.; Kato, T.; Kobayashi, T.; Koizumi, T.; et al. In Vivo Administration of Recombinant Human Granulocyte Colony-Stimulating Factor Increases the Immune Effectiveness of Dendritic Cell-Based Cancer Vaccination. Vaccines 2019, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Koya, T.; Yanagisawa, R.; Higuchi, Y.; Sano, K.; Shimodaira, S. Interferon-α-Inducible Dendritic Cells Matured with OK-432 Exhibit TRAIL and Fas Ligand Pathway-Mediated Killer Activity. Sci. Rep. 2017, 7, 42145. [Google Scholar] [CrossRef] [Green Version]
- Spadaro, F.; Lapenta, C.; Donati, S.; Abalsamo, L.; Barnaba, V.; Belardelli, F.; Santini, S.M.; Ferrantini, M. IFN-α Enhances Cross-Presentation in Human Dendritic Cells by Modulating Antigen Survival, Endocytic Routing, and Processing. Blood 2012, 119, 1407–1417. [Google Scholar] [CrossRef] [Green Version]
- Papewalis, C.; Jacobs, B.; Wuttke, M.; Ullrich, E.; Baehring, T.; Fenk, R.; Willenberg, H.S.; Schinner, S.; Cohnen, M.; Seissler, J.; et al. IFN-Alpha Skews Monocytes into CD56+-Expressing Dendritic Cells with Potent Functional Activities In Vitro and In Vivo. J. Immunol. Baltim. 2008, 180, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Rozera, C.; Cappellini, G.A.; D’Agostino, G.; Santodonato, L.; Castiello, L.; Urbani, F.; Macchia, I.; Aricò, E.; Casorelli, I.; Sestili, P.; et al. Intratumoral Injection of IFN-Alpha Dendritic Cells after Dacarbazine Activates Anti-Tumor Immunity: Results from a Phase I Trial in Advanced Melanoma. J. Transl. Med. 2015, 13, 139. [Google Scholar] [CrossRef]
- Cox, M.C.; Castiello, L.; Mattei, M.; Santodonato, L.; D’Agostino, G.; Muraro, E.; Martorelli, D.; Lapenta, C.; Di Napoli, A.; Di Landro, F.; et al. Clinical and Antitumor Immune Responses in Relapsed/Refractory Follicular Lymphoma Patients after Intranodal Injections of IFNα-Dendritic Cells and Rituximab: A Phase I Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 5231–5241. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Wu, X.Y.; Tong, J.B.; Yang, X.X.; Zhao, J.L.; Zheng, Q.F.; Zhao, G.B.; Ma, Z.J. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue under Xeno-Free Conditions for Cell Therapy. Stem Cell Res. Ther. 2015, 6, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Bonin, M.; Stölzel, F.; Goedecke, A.; Richter, K.; Wuschek, N.; Hölig, K.; Platzbecker, U.; Illmer, T.; Schaich, M.; Schetelig, J.; et al. Treatment of Refractory Acute GVHD with Third-Party MSC Expanded in Platelet Lysate-Containing Medium. Bone Marrow Transplant. 2009, 43, 245–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, G.; Fleck, E.; Elser, S.; Hermanutz-Klein, U.; Waidmann, M.; Northoff, H.; Seifried, E.; Schäfer, R. Manufacture of Endothelial Colony-Forming Progenitor Cells from Steady-State Peripheral Blood Leukapheresis Using Pooled Human Platelet Lysate. Transfusion 2018, 58, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Daniliuc, S.; Genser-Nir, M.; Miropolski, Y.; Sharovetsky, M.; Hazan Brill, R.H.; Fiorentini, D.; McNiece, I.; Kellner, J. Cell Therapy-Compliant Xeno-Free Culture System for Human Endothelial Cells. Cytotherapy 2018, 20, S86. [Google Scholar] [CrossRef]
- Fekete, N.; Gadelorge, M.; Fürst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailänder, V.; Lotfi, R.; Ignatius, A.; Sensebé, L.; et al. Platelet Lysate from Whole Blood-Derived Pooled Platelet Concentrates and Apheresis-Derived Platelet Concentrates for the Isolation and Expansion of Human Bone Marrow Mesenchymal Stromal Cells: Production Process, Content and Identification of Active Components. Cytotherapy 2012, 14, 540–554. [Google Scholar] [CrossRef] [Green Version]
- Švajger, U. Human Platelet Lysate Is a Successful Alternative Serum Supplement for Propagation of Monocyte-Derived Dendritic Cells. Cytotherapy 2017, 19, 486–499. [Google Scholar] [CrossRef]
- Kral, J.B.; Schrottmaier, W.C.; Salzmann, M.; Assinger, A. Platelet Interaction with Innate Immune Cells. Transfus. Med. Hemother. 2016, 43, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Shimodaira, S.; Koya, T.; Higuchi, Y.; Okamoto, M.; Koido, S. Quality Verification of Dendritic Cell-Based Cancer Vaccine. Pharm. Anal. Acta 2015, 7. [Google Scholar] [CrossRef]
- Korthals, M.; Safaian, N.; Kronenwett, R.; Maihöfer, D.; Schott, M.; Papewalis, C.; Diaz Blanco, E.; Winter, M.; Czibere, A.; Haas, R.; et al. Monocyte Derived Dendritic Cells Generated by IFN-α Acquire Mature Dendritic and Natural Killer Cell Properties as Shown by Gene Expression Analysis. J. Transl. Med. 2007, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Tekkatte, C.; Gunasingh, G.; Cherian, K.; Sankaranarayanan, K. “Humanized” Stem Cell Culture Techniques: The Animal Serum Controversy. Stem Cells Int. 2011, 2011, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Presciutti, S.M.; Paglia, D.N.; Karukonda, T.; Soung, D.Y.; Guzzo, R.; Drissi, H.; Moss, I.L. PDGF-BB Inhibits Intervertebral Disc Cell Apoptosis in Vitro. J. Orthop. Res. 2014, 32, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.Y.; Chao, Y.W.; Tsai, Y.L.; Lien, C.C.; Chang, C.F.; Deng, M.C.; Ho, L.T.; Kwok, C.F.; Juan, C.C. Resistin Induces Monocyte–Endothelial Cell Adhesion by Increasing ICAM-1 and VCAM-1 Expression in Endothelial Cells via P38MAPK-Dependent Pathway. J. Cell. Physiol. 2011, 226, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes Derived from Platelet-Rich Plasma Present a Novel Potential in Alleviating Knee Osteoarthritis by Promoting Proliferation and Inhibiting Apoptosis of Chondrocyte via Wnt/β-Catenin Signaling Pathway. J. Orthop. Surg. 2019, 14, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudek, A.M.; Martin, S.; Garg, A.D.; Agostinis, P. Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front. Immunol. 2013, 4, 438. [Google Scholar] [CrossRef] [Green Version]
- Rutella, S.; Danese, S.; Leone, G. Tolerogenic Dendritic Cells: Cytokine Modulation Comes of Age. Blood 2006, 108, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Barratt-Boyes, S.M.; Zimmer, M.I.; Harshyne, L.A.; Meyer, E.M.; Watkins, S.C.; Capuano, S.; Murphey-Corb, M.; Falo, L.D.; Donnenberg, A.D. Maturation and Trafficking of Monocyte-Derived Dendritic Cells in Monkeys: Implications for Dendritic Cell-Based Vaccines. J. Immunol. 2000, 164, 2487–2495. [Google Scholar] [CrossRef] [Green Version]
- Barratt-Boyes, S.M.; Watkins, S.C.; Finn, O.J. In Vivo Migration of Dendritic Cells Differentiated In Vitro: A Chimpanzee Model. J. Immunol. 1997, 158, 4543–4547. [Google Scholar]
- Padovan, E.; Spagnoli, G.C.; Ferrantini, M.; Heberer, M. IFN-alpha2a Induces IP-10/CXCL10 and MIG/CXCL9 Production in Monocyte-Derived Dendritic Cells and Enhances Their Capacity to Attract and Stimulate CD8+ Effector T Cells. J. Leukoc. Biol. 2002, 71, 669–676. [Google Scholar] [CrossRef]
- Muthuswamy, R.; Mueller-Berghaus, J.; Haberkorn, U.; Reinhart, T.A.; Schadendorf, D.; Kalinski, P. PGE2 Transiently Enhances DC Expression of CCR7 but Inhibits the Ability of DCs to Produce CCL19 and Attract Naive T Cells. Blood 2010, 116, 1454–1459. [Google Scholar] [CrossRef]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.X.; et al. PD-L1 on Dendritic Cells Attenuates T Cell Activation and Regulates Response to Immune Checkpoint Blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 Regulates the Development, Maintenance, and Function of Induced Regulatory T Cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Hanlon, D.; Arshad, N.; Lee, J.S.; Tatsuno, K.; Robinson, E.; Filler, R.; Sobolev, O.; Cote, C.; Rivera-Molina, F.; et al. Platelet P-Selectin Initiates Cross-Presentation and Dendritic Cell Differentiation in Blood Monocytes. Sci. Adv. 2020, 6, eaaz1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, H.L.; Bancroft, G.J. Cytokine Enhancement of Complement-Dependent Phagocytosis by Macrophages: Synergy of Tumor Necrosis Factor-α and Granulocyte-Macrophage Colony-Stimulating Factor for Phagocytosis of Cryptococcus neoformans. Eur. J. Immunol. 1992, 22, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I. Mechanisms Controlling EGF Receptor Endocytosis and Degradation. Biochem. Soc. Trans. 2003, 31, 1178–1181. [Google Scholar] [CrossRef]
- Ming, J.E.; Steinman, R.M.; Granelli-Piperno, A. IL-6 Enhances the Generation of Cytolytic T Lymphocytes in the Allogeneic Mixed Leucocyte Reaction. Clin. Exp. Immunol. 1992, 89, 148–153. [Google Scholar] [CrossRef]
- Mosser, D.M.; Zhang, X. Interleukin-10: New Perspectives on an Old Cytokine. Immunol. Res. 2008, 226, 205–218. [Google Scholar] [CrossRef]
- Santin, A.D.; Hermonat, P.L.; Ravaggi, A.; Bellone, S.; Pecorelli, S.; Roman, J.J.; Parham, G.P.; Cannon, M.J. Interleukin-10 Increases Th1 Cytokine Production and Cytotoxic Potential in Human Papillomavirus-Specific CD8+ Cytotoxic T Lymphocytes. J. Virol. 2000, 74, 4729–4737. [Google Scholar] [CrossRef] [Green Version]
- Fujii, S.; Shimizu, K.; Shimizu, T.; Lotze, M.T. Interleukin-10 Promotes the Maintenance of Antitumor CD8+ T-Cell Effector Function In Situ. Blood 2001, 98, 2143–2151. [Google Scholar] [CrossRef] [Green Version]
- Scheurich, P.; Thoma, B.; Ucer, U.; Pfizenmaier, K. Immunoregulatory Activity of Recombinant Human Tumor Necrosis Factor (TNF)-Alpha: Induction of TNF Receptors on Human T Cells and TNF-Alpha-Mediated Enhancement of T Cell Responses. J. Immunol. Baltim. MD 1950, 138, 1786–1790, 1987. [Google Scholar]
- Del Vecchio, M.D.; Bajetta, E.; Canova, S.; Lotze, M.T.; Wesa, A.; Parmiani, G.; Anichini, A. Interleukin-12: Biological Properties and Clinical Application. Clin. Cancer Res. 2007, 13, 4677–4685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobl, H.; Knapp, W. TGF-Β1 Regulation of Dendritic Cells. Microbes Infect. 1999, 1, 1283–1290. [Google Scholar] [CrossRef]
- Clausen, B.E.; Stoitzner, P. Functional Specialization of Skin Dendritic Cell Subsets in Regulating T Cell Responses. Front. Immunol. 2015, 6, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klechevsky, E.; Morita, R.; Liu, M.; Cao, Y.; Coquery, S.; Thompson-Snipes, L.; Briere, F.; Chaussabel, D.; Zurawski, G.; Palucka, A.K.; et al. Functional Specializations of Human Epidermal Langerhans Cells and CD14+ Dermal Dendritic Cells. Immunity 2008, 29, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Date, I.; Koya, T.; Sakamoto, T.; Togi, M.; Kawaguchi, H.; Watanabe, A.; Kato, T., Jr.; Shimodaira, S. Interferon-α-Induced Dendritic Cells Generated with Human Platelet Lysate Exhibit Elevated Antigen Presenting Ability to Cytotoxic T Lymphocytes. Vaccines 2021, 9, 10. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9010010
Date I, Koya T, Sakamoto T, Togi M, Kawaguchi H, Watanabe A, Kato T Jr., Shimodaira S. Interferon-α-Induced Dendritic Cells Generated with Human Platelet Lysate Exhibit Elevated Antigen Presenting Ability to Cytotoxic T Lymphocytes. Vaccines. 2021; 9(1):10. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9010010
Chicago/Turabian StyleDate, Ippei, Terutsugu Koya, Takuya Sakamoto, Misa Togi, Haruhiko Kawaguchi, Asuka Watanabe, Tomohisa Kato, Jr., and Shigetaka Shimodaira. 2021. "Interferon-α-Induced Dendritic Cells Generated with Human Platelet Lysate Exhibit Elevated Antigen Presenting Ability to Cytotoxic T Lymphocytes" Vaccines 9, no. 1: 10. https://0-doi-org.brum.beds.ac.uk/10.3390/vaccines9010010