1. Introduction
Microplastics (MPs), pharmaceutical compounds and perfluorinated compounds (PFCs) such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) are persistent organic pollutants of emerging concern [
1,
2]. The occurrence of the aforementioned compounds poses a potential threat of toxicity to the biotic and abiotic ecosystem [
3]. Due to the environmental concerns instigated by water pollution as a result of rapid population growth and industrialisation which has a direct impact on wastewater composition which is now characterised by contaminants of emerging concern (PFCs, pharmaceuticals, MPs, heavy metal ions, etc.,) which have eluded conventional wastewater treatment processes [
4,
5]. This is attributed to the fact that conventional wastewater treatment processes are designed to treat wastewater streams characterised with organic pollutants such as chemical oxygen demand, total dissolved solids, biological nutrients, e.g., phosphates and nitrates, etc., and they have demonstrated to be ineffective to handle the current wastewater volumes [
5,
6,
7].
PFCs, which consist of the carbon-fluorine bond (C-F), are characterised by strong polarity and strength, which is attributed by the strong electro negativity of fluorine not only in halogens but in all elements in the periodic table [
1,
8]. Due to the aforementioned properties, PFCs have cemented their application in a variety of products and industries. Moreover, these PFCs are resistant to degradation by heat and acid [
8,
9,
10,
11]. PFCs are widely used for surface treatment of textiles due to their repulsion behaviour in both water and oil [
8], used as a formulation agent of aqueous film forming foams commonly used for combating hydrocarbon fires [
7,
8], and used as food packing paper and in leather treatment [
9,
10], polymer emulsifier and insecticides [
12]. PFCs are characterised as a class of emerging persistent organic pollutants that consist of a fully fluorinated hydrophobic alkyl chain attached to a hydrophilic chain end group, as depicted in
Figure 1 [
9,
10]. The occurrence of PFCs in surface water, i.e., rivers, ponds and lakes is attributed to the discharge of untreated industrial effluent, leakage from the soil as well as surface deposition [
7]. Due to long term exposure to PFCs, several studies [
7,
11,
13] have reported that some PFCs, particularly PFOAs and PFOSs, have been detected in human tissue and blood serum. Crone et al. [
13] and EPA [
14] have reported the human health complications associated with exposure to PFOAs and PFOSs which include high cholesterol, increased liver enzymes, testicular and kidney cancer, poor vaccination response, thyroid disorders, pregnancy-induced hypertension and preeclampsia, immune suppression as well as reduced fertility.
Pharmaceuticals such as sulfamethazine (SMT), which is characterised as an active drug compound and classified as an antibiotic, has been detected in the environment [
15]. This may be attributed to the application of SMT for a wide range of antibacterial activity, high therapeutic effectiveness and low costs [
15,
16]. Apart from the usefulness of SMT, its occurrence in the environment poses a serious health risk to the aquatic ecosystem, flora, fauna and humans, as reported in previous studies [
15,
17,
18]. On the other hand, the occurrence of plastic residues in surface water is an emerging environmental problem that is attributed to the unsustainable disposal of plastic residues, which ultimately reaches and persists in the aquatic environment [
19,
20,
21]. According to Wu et al. [
20], these plastic residues can undergo processes of degradation, embrittlement and fragmentation when they are subjected to certain conditions such as solar radiation, wave slap, and temperature change as well as biological effects. Upon their degradation, once the size of these synthetic polymers is less than 5 mm without any lower limit, they are called microplastics [
20,
22]. The hazardous effects of MPs are not limited to their ability to release hazardous organic monomers [
23], but also include their ability to interact with other pollutants [
20], thus posing a high health risk to aquatic life. It should be noted that MPs with or without pollutants can be ingested by aquatic life, thus resulting in bioaccumulation, which can consistently adversely affect numerous organs since they are characterised as endocrine disrupting compounds [
20,
24].
Until now, researchers have been exploring new avenues aimed at designing effective water and wastewater treatment processes aimed at combating the bioaccumulation of emerging contaminants on the environment. Solid–liquid adsorption has drawn so much attention for many researchers as a promising technology to combat the occurrence of pollutants in the environment [
25,
26]. The purpose of this paper is to study the adsorption mechanisms of PFCs and SMT by investigating the thermodynamic interaction of PFOA, PFOS and SMT in polyethylene (PE) and polypropylene (PP) through phase equilibrium and mixing using the extended Flory–Huggins approach in Material Studio. To the best of our knowledge, there are no reported studies on investigating the effect of temperature and polymerisation on the thermodynamic interaction of the aforementioned compounds with PE and PP using the extended Flory–Huggins approach. The findings of the current study will contribute to the design of solid–liquid adsorption wastewater treatment processes aimed at eradicating the occurrence of the model contaminants, thus improving the water quality. It should be noted that PE and PP are the most frequently detected types of MPs in the aquatic environment [
27].
2. Materials and Methods
The simplest thermodynamic theory to describe mixing and phase separation is the Flory–Huggins approach [
28], which describes the free energy of mixing (Δ
G) in binary systems as follows:
Wherein
φi is the volume fraction of component
i,
ni is the degree of polymerisation of component
i,
χbs is the binary interaction parameter, and
T and
R are the temperature and universal gas constant, respectively. The subscripts
b and
s refer to the role of the component in question in the mixture, namely whether it is the base (
b) or the screen (
s). The mixing energy
Emix is used to determine the interaction parameter
χbs:
The conventional Flory–Huggins model employs a lattice description for each component. In the case of a lattice with the coordination number set to
Z, the mixing energy is then a function of the binding energy
Eij between each component pair
ij:
The Flory–Huggins approach was extended by incorporating techniques from molecular simulations, specifically by considering the binding energies as averages over an ensemble of molecular configurations [
29,
30] as implemented in version 2020 of the computer program Materials Studio [
31]. Firstly, the interaction parameter’s temperature dependence is described explicitly by generating a large number of pair configurations and calculating the associated binding energies, followed by averaging results via the Boltzmann factor and determining the resulting temperature-dependent interaction parameter. Secondly, an off-lattice structure is considered, by which the coordination number
Z is computed explicitly for each of the possible configurations using atomistic molecular simulations. Use of a temperature-dependent interaction parameter
χ in the Flory–Huggins expression yields the free energy for all compositions and temperatures, following which the phase diagram can be determined by computing the critical point, binodal, and spinodal curves.
The binding energies given in Equation (3) were calculated by sampling from energetically favourable configurations by way of the volume constraint method [
29], wherein molecules are combined in order for their van der Waals surfaces to enter into contact with each other. Depending on the roles of the pair of molecules under consideration, the binding energy is yielded for each of the binding energy combinations
Ebs,
Esb,
Ebb, and
Ess. It should be noted that
Ebs and
Esb are equivalent, and so there are three binding energy pairs that are actually computed. To calculate the binding energy, two molecular structures representing the condensed state are generated, and if a molecule possesses significant flexibility, then a set of conformations is generated which is presentative of the condensed state. A single seed molecule is first introduced into the system following which a packing molecule is positioned such that its centre of mass is located at the same position as the original seed molecule. Next, the packing molecule’s rotation is altered randomly, and it is then translated along a randomly selected direction until its van der Waals surface ceases to overlap but remains in contact with the initial seed molecule. The pairwise interaction energy of the resulting configuration is stored, and a histogram (with a bin width of 8 × 10
−2 kJ/mol) of the resulting distribution of the binding energy is derived from 10
7 energy samples. The average weighted binding energy <
Eij>
T at a specified temperature
T is computed as the weighted average of a distribution function using the Boltzmann factor exp (−
Eij/
RT):
The coordination number Z describes the number of molecules of one component that can be packed around a solitary molecule of the other species in the pair under consideration. A single molecule of component i surrounded by Zij molecules of component j together constitute a cluster consisting of a single seed molecule of i with Zij packing molecules. The four different combinations of clusters that are considered (using the aforementioned base-screen terminology) are as follows: Zbb (in which the base species plays the role of both seed and packing molecules), Zbs (for which the base species is the seed, and the screen molecules are the packing), Zsb (the converse of Zbs), and Zss (wherein the screen species is both the seed and the packing). To determine the coordination number, the approach used to estimate the binding energy was extended (neglecting the energy calculation itself). First, a single seed molecule was introduced, following which a packing molecule was added such that its centre of mass was overlaid onto the centre of mass of the initial seed molecule. The packing molecule was then rotated a random amount, following which it was translated in a random direction until its van der Waals surface no longer overlapped with the seed molecule. Subsequent packing molecules were added in a similar fashion, although with overlapping being avoided for the newly added packing molecule with respect to the cluster thus far (i.e., the seed + packing molecules already in the system). Furthermore, any newly introduced packing molecules must be in contact with the seed molecule; if they are not, their introduction into the system is rejected and another attempt is made to add a packing molecule. These steps are repeated for 20 iterations per cluster type and 105 samples per cluster to determine the average coordination number.
Once the weighted average binding energies <
Eij>
T and average coordination numbers
Zij are computed, the mixing energy was calculated via:
The binary interaction parameter
χbs is then readily computed as
Emix/
RT for subsequent use within Flory–Huggins theory as outlined previously. Once the free energy isotherms are known, the phase diagram can be computed. The implementation of the extended Flory–Huggins approach employed in this study included a search for temperature ranges for which phase separation occurs, which may be noted as a limitation of the study. At the critical point (where the two phases become indistinguishable), both the second and third derivatives of the free energy with respect to composition disappear. The coexistence region is bounded by the binodal curve, which indicates the conditions at which two distinct separate phases may coexist; in the case of the systems under consideration in the study, this implies the existence of a PFC-in-polymer phase in conjunction with a polymer-in-PFC phase. In practice, the concentration of the polymer in the polymer-in-PFC phase is so low that it is negligible, as the polymers are largely not dissolved into the PFC, whereas the PFC is absorbed into the polymeric matrix. The binodal curve connects the distant points on either side of the critical point, at which the first derivative of the free energy is equal to the ratio of the free energy difference with respect to the composition difference:
where
xi is the binodal composition of phase
i. The locations within the coexistence region where the second derivatives of the free energy with respect to the compositions are zero (i.e., inflection points) yields the spinodal curve which describes the point at which phase separation occurs.
The approach of describing sorption in polymers by way of phase equilibrium and mixing is analogous to previous work on water absorption in polyethylene [
32,
33] and polyterafluoroethylene [
34].
3. Results and Discussion
Commercial plastics are characterised by synthetic polymers derived from fossil-based sources, such as PE and PP, amongst others [
35], where PP and PE are the most detected synthetic polymers in the environment. The findings of the current study on the interaction between MPs and the model contaminants of emerging concern are interpreted with reference to three main sorption mechanisms, i.e., pore-filling, hydrophobic interaction, and van der Waals forces [
36,
37].
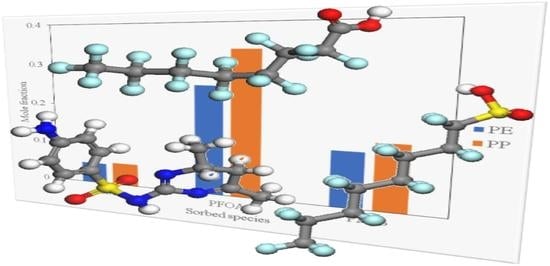
Figure 2,
Figure 3 and
Figure 4 show results for the sorption of all studied chemical contaminants on PE and PP. The results are presented in terms of temperature variation for different degrees of polymerisation for each polymer investigated. The standard deviation derived from three independent computations is estimated at about 0.05%, which is too small to display on the diagrams. It is apparent that there is a strong correlation between temperature and the degree of sorption, whilst the degree of polymerisation has a limited effect, at least in terms of molar composition. The pore-filling sorption mechanism as characterised by Wang et al. [
23] involves the process of contaminants entering the polymer pores and remaining trapped inside the nano-scale pores because of the many pores of different sizes that exist in polymers. This sorption mechanism is highly temperature-dependent. It should be noted that when heat energy is applied to a polymer molecular chain, it allows the polymer to expand. At the glass transition temperature, which is the temperature at which 30–50 carbon chains start to move [
38], the amorphous region dissociates from a rigid state to a rubbery state, thus, increasing the free volume which is basically the gap between molecular chains, for the sorption of contaminants. While not directly accounted for in this work’s methodology, the observed results may be attributed to PE and PP being rubbery plastics, as indicated in previous studies [
23,
39].
PP recorded higher contaminant sorption at lower temperatures when compared to PE for all contaminant species. This is attributed to the difference in molecular weight between PP (-CH
2-CH
2-CH
2-)
n and PE (-CH
2-CH
2-)
n; according to Young and Lovell [
40], there is a strong correlation in the increase in polymer molecular weight and the total fractional free volume of a polymer, consequently favouring the pore-filling sorption mechanism of contaminants. While not explicitly accounted for in the computations in this study, considering the effect of polymer chain length on the glass transition temperature can be instructive. It should be noted that the glass transition temperature increases with the increase in polymer molecular weight, yet the findings of the current study indicate that sorption of PFOA, PFOS and SMT onto PP occurred at a lower temperature compared to that for PE. This can be attributed to PE having higher crystallinity as compared to PP, thus requiring more thermal energy to expand the PE polymer, consequently increasing its free volume allowing for pore-filling sorption to occur at relatively high temperatures compared to PP. The observed results suggest that this effect or an analogous one may be implicitly produced in the course of the computations employed in this study.
The sorption of PFOS and PFOA on PP and PE at low temperature conditions of 300 K as depicted in
Figure 2 and
Figure 3 may be attributed to van der Waals force interaction. This is a relatively weak force characterising the attraction of intermolecular forces between MP molecules (i.e., PP and PE) and organic contaminant molecules, thus allowing them to adhere to each other as reported by Wang et al. [
23] and Agboola and Benson [
41]. Based on previous works [
23,
41,
42], it is apparent that the sorption of aliphatic as well as aromatic organic contaminants into aliphatic MPs can also be interpreted by van der Waals force interactions. Moreover, Xu et al. [
43] reported a linear relationship for the sorption of sulfamethoxazole on PE. However, it was reported that under the pH conditions in which the study was conducted, both sulfamethoxazole and PE, MPs recorded negative charges, hence the sorption mechanism could not be explained by hydrophobic or electrostatic interaction. For the current study, based on the findings presented in
Figure 4, it is apparent that there was significant sorption of SMT at temperature conditions of 450 K and above on both PE and PP. The sorption mechanism of SMT on PE and PP may be attributed to pore-filling. It is imperative to note that SMT sorption on PE and PP is highly temperature dependant which may be attributed to the molecular structure of SMT (C
12H
14N
4O
2S), hence an increase in temperature allows for the motion of molecules for MPs consequently increasing the free volume to allow for the sorption of SMT.
Figure 2 shows that PFOA demonstrated high sorption affinity towards PP and PE when compared to PFOS (see
Figure 3). This may be attributed to the difference in physicochemical properties of the two compounds; PFOA is characterised as a non-polar compound, whereas PFOS exhibits some polarity because of the sulfonate group. It should be noted that C-C as well as C-H bonds in hydrocarbons are characterised as non-polar molecules, hence PE and PP are non-polar polymers. Due to the non-polarity of PE and PP, PFOA demonstrated high sorption affinity towards PE and PP by the polar-polar interaction as compared to PFOS which exhibited some degree of polarity.
In addition to examining sorption for pure contaminant species, it is instructive to include the results for water since the conditions of interest for PFOA, PFOS and SMT are aqueous environments. These results are presented in
Figure 5. Direct comparisons between water and the contaminant species at a fixed polymerisation of Np = 100 are shown in
Figure 6 and
Figure 7 for PE and PP, respectively. In this context, it is apparent that both PE and PP are likely to not be useful to remove SMT in aqueous conditions, since water displays higher affinity for sorption in both polymer species than SMT. Both PFOS and PFOA exhibit stronger affinity for uptake in PE and PP than water. This is not entirely explained by the polarity of PFOS and PFOA with respect to water, since (within the framework of the COMPASS force field) the respective magnitudes of the dipole moments are 3.06, 1.95, and 2.30 Debye, respectively (it should be noted that the magnitude of the dipole moment of SMT is 3.85 Debye). As organic molecules, PFOS and PFOA do, of course, possess carbon chains, which would interact with polymer chains even if the molecules as a whole are significantly polar. Hence, the interactions between the contaminant molecules and polymers (especially when considered within an aqueous environment) require a more nuanced assessment. In terms of the temperature effects, at ambient conditions (i.e., around 298 K), it is apparent that only PFOA has higher sorption in both PE and PP than water. Meanwhile, higher temperatures are necessary to induce preferential uptake of PFOS in aqueous environments (above 390 K for PP, and over 450 K for PE), which shows that these MPs may be vectors for transporting PFOA into the environment in general, whilst special conditions are required for environmental transmission of PFOS in this manner. It is then also unlikely that PE and PP are vectors for the transportation of SMT into aqueous environments. This implies that both PE and PP are transport vectors for PFOA as well as posing a risk for bioaccumulation in marine animals via ingestion of MPs, causing negative health effects as reported by Wu et al. [
20] and Ramhøj et al. [
24].
In addition to demonstrating that PE and PP are transport vectors for PFOA, this work suggests that these materials may be useful to remove said compounds from aqueous environments, when employed in a controlled scenario such as water treatment. Due to the propensity for PFOA uptake in both PE and PP, it is conceivable that polymer pellets could be used to remove this compound from wastewater to prevent bioaccumulation in the environment. As for SMT and PFOS, alternative removal techniques may be necessary, since PE and PP may only offer targeted removal of PFOA. Expanding on the work of this study may yield additional useful observations which suggest other common materials to remove SMT and PFOS from wastewater and may assist in the design of new water treatment processes to remove these pharmaceutical compounds from the environment.
It is worth noting that at the time this study was conducted, they were no studies reported on investigating the sorption behaviour of SMT, PFOA and PFOS relative to water, PE and PP using the extended Flory–Huggins approach. Nor are there reported studies on the effect of degree of polymerisation and temperature variation on the sorption behaviour of the above-mentioned model contaminants relative to water and polymer. However, there are reported studies focusing on solid–liquid sorption of perfluorinated compounds in the aqueous environment. Lee et al. [
44] investigated the sorption of PFOA, perfluoro-n-pentanoic acid (PFPeA), perfluorohexane sulfonate (PFHxS), perfluorohexanoic acid (PFHxA), and perfluorononaoic acid (PFNA) in the aqueous environment relative to activated carbon. Lee and co-workers reported that 90% removal of the aforementioned model PFCs was achieved. Meng et al. [
45] evaluated the sorption efficiency of magnetic activated carbon on the removal of PFOS, PFOA, PFHxS and perfluorobutane sulfonate (PFBS) in aqueous solution and the observed affinity was PFOS > PFOA > PFHxS > PFBS with adsorption equilibriums of 1.63, 0.90, 0.33 and 0.21 mmol/g, respectively. The findings observed by Meng et al. [
45] when compared to the current study suggest that activated carbon has a higher affinity towards PFOS compared to PE and PP. This sorption pattern can be attributed on the fact that activated carbon has a higher affinity towards organic compounds that are more hydrophobic and less polar [
46].
4. Conclusions and Future Perspectives
An extended Flory–Huggins approach was used to model sorption of SMT, PFOA and PFOS on PE and PP. Previous work [
29,
30,
31] using Gibbs ensemble Monte Carlo simulations modelled sorption in polymers by considering phase equilibria between polymers and fluid systems, analogous to the current work undertaken along different methodological lines. For the sake of reference within the context of aqueous environments, sorption of water in both polymers was also considered. Both temperature and the degree of polymerisation were considered, and it was found that on a molar basis the latter did not play a significant role in the uptake of SMT, PFOA, PFOS or water, whereas temperature effects were significant. While not explicitly accounted for in this work, the results were evocative of a pore-filling mechanism.
Based on the findings of the current study, it is apparent that at ambient conditions, only the sorption of PFOA on both PE and PP was favoured in aqueous environments, while higher temperatures could induce uptake of PFOS into both polymers. This implies that PE and PP are not only transport vectors for PFOA into the environment at large, but also that they pose a risk for ingestion by marine or aquatic animals resulting in bioaccumulation of PFOA, causing serious negative health effects as reported by Wu et al. [
20] and Ramhøj et al. [
23]. Sorption of SMT into both polymers was not preferential compared to water at any conditions investigated in this study, suggesting that PE and PP are probably not significant vectors for transportation of SMT into aquatic or marine environments.
To date, adequate studies have been published on the occurrence and concentration levels of PFCs and pharmaceuticals in water receiving bodies with the application of liquid-solid adsorption as a promising technique. However, the is no information available on the interaction of PFCs, pharmaceuticals with polymers, i.e., MPs in aqueous environments since these contaminants coexist. It should be noted that the occurrence and bioaccumulation of polymers by aquatic life in the form of MPs have become a global phenomenon. Therefore, it is imperative to understand the interaction mechanisms of MPs with the aforementioned contaminants in order to develop effective tools aiming to detect and eradicate these emerging contaminants in water bodies. Information on the thermodynamic interaction of PFCs and pharmaceuticals relative to MPs will give an insight into understanding the potential of MPs serving as a transport vector for organic contaminants.
It is appreciated that several studies have been conducted aimed at removing PFCs and pharmaceuticals from aqueous streams using both biological and liquid-solid adsorption separation. However, further studies are needed to investigate the application of polymers as remediation technology for PFCs and pharmaceuticals using raw wastewater aimed at investigating the effect of other organic pollutants on polymer affinity towards PFCs and pharmaceuticals. Moreover, the current study does not account for pH variation and the occurrence of co-pollutants in polymer structures on polymer affinity towards PFCs and pharmaceuticals.