Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
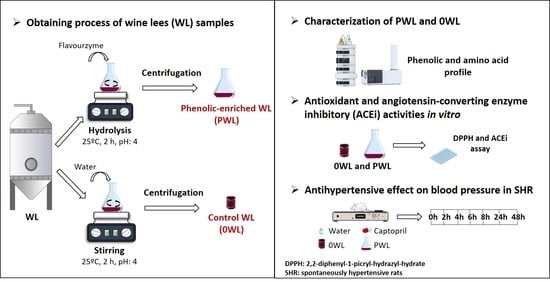
2.2. Preparation of the Wine Lees Hydrolysate
2.3. Characterization of Wine Lees
2.4. Identification and Quantification of the Phenolic Profile
2.5. Antioxidant Activity
2.6. ACE Inhibitory Activity
2.7. Antihypertensive Effect
2.8. Statistical Analysis
3. Results
3.1. Wine Lees Composition
3.2. Determination of the Phenolic Profile in Wine Lees Samples
3.3. Antioxidant and ACEi Activities of the Wine Lees
3.4. Antihypertensive Activity of the Wine Lees
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, B.; Li, F.; Wang, L.; Zhang, D. Fruit and Vegetables Consumption and Risk of Hypertension: A Meta-Analysis. J. Clin. Hypertens 2016, 18, 468–476. [Google Scholar] [CrossRef]
- Adebawo, O.; Salau, B.; Ezima, E.; Oyefuga, O.; Ajani, E.; Idowu, G.; Famodu, A.; Osilesi, O. Fruits and Vegetables Moderate Lipid Cardiovascular Risk Factor in Hypertensive Patients. Lipids Health Dis. 2006, 5, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alissa, E.M.; Ferns, G.A. Dietary Fruits and Vegetables and Cardiovascular Diseases Risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S. [Google Scholar] [CrossRef] [PubMed]
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in Human Nutrition: From the In Vitro Antioxidant Capacity to the Beneficial Effects on Cardiometabolic Health and Related Inter-Individual Variability—An Overview and Perspective. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spagnuolo, C.; Russo, M.; Bilotto, S.; Tedesco, I.; Laratta, B.; Russo, G.L. Dietary Polyphenols in Cancer Prevention: The Example of the Flavonoid Quercetin in Leukemia. Ann. N. Y. Acad. Sci. 2012, 1259, 95–103. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Grape Seed Flavanols Decrease Blood Pressure via Sirt-1 and Confer a Vasoprotective Pattern in Rats. J. Funct. Foods 2016, 24, 164–172. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Ky, I.; Cros, G.; Crozier, A.; Teissedre, P.-L. Grape Pomace: Antioxidant Activity, Potential Effect against Hypertension and Metabolites Characterization after Intake. Diseases 2018, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Costa, G.F.; Ognibene, D.T.; Da Costa, C.A.; Teixeira, M.T.; Da Silva Cristino Cordeiro, V.; De Bem, G.F.; Moura, A.S.; De Castro Resende, A.; De Moura, R.S. Vitis Vinifera L Grape Skin Extract Prevents Development of Hypretension and Altered Lipid Profile in Sponteneously Hypertensive Tats: Role of Oxidative Stress. Prev. Nutr. Food Sci. 2020, 25, 25–31. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of Angiotensin-Converting Enzyme Activity by Flavonoids: Structure-Activity Relationship Studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [Green Version]
- Foëx, P.; Sear, J. Hypertension: Pathophysiology and Treatment. Contin. Educ. Anaesth. Crit. Care Pain 2004, 4, 71–75. [Google Scholar] [CrossRef]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin System in Kidney Physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [PubMed] [Green Version]
- Snauwaert, E.; Vande Walle, J.; De Bruyne, P. Therapeutic Efficacy and Safety of ACE Inhibitors in the Hypertensive Paediatric Population: A Review. Arch. Dis. Child. 2017, 102, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alderman, C.P. Adverse Effects of the Angiotensin-Converting Enzyme Inhibitors. Ann. Pharm. 1996, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Natural Angiotensin Converting Enzyme (ACE) Inhibitors with Antihypertensive Properties. In Natural Products Targeting Clinically Relevant Enzymes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 45–67. [Google Scholar]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- López-Fernández-Sobrino, R.; Soliz-Rueda, J.R.; Margalef, M.; Arola-Arnal, A.; Suárez, M.; Bravo, F.I.; Muguerza, B. ACE Inhibitory and Antihypertensive Activities of Wine Lees and Relationship among Bioactivity and Phenolic Profile. Nutrients 2021, 13, 679. [Google Scholar] [CrossRef]
- Queiroz, M.; Oppolzer, D.; Gouvinhas, I.; Silva, A.M.; Barros, A.I.R.N.A.; Domínguez-Perles, R. New Grape Stems’ Isolated Phenolic Compounds Modulate Reactive Oxygen Species, Glutathione, and Lipid Peroxidation In Vitro: Combined Formulations with Vitamins C and E. Fitoterapia 2017, 120, 146–157. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-Molecular Procyanidin Rich Grape Seed Extract Exerts Antihypertensive Effect in Males Spontaneously Hypertensive Rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J.D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein-Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Radenkovs, V.; Juhnevica-Radenkova, K.; Górnaś, P.; Seglina, D. Non-Waste Technology through the Enzymatic Hydrolysis of Agro-Industrial by-Products. Trends Food Sci. Technol. 2018, 77, 64–76. [Google Scholar] [CrossRef]
- Landbo, A.K.; Meyer, A.S. Enzyme-Assisted Extraction of Antioxidative Phenols from Black Currant Juice Press Residues (Ribes Nigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Enzyme-Enhanced Extraction of Phenolic Compounds and Proteins from Flaxseed Meal. ISRN Biotechnol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and Future Strategies for Wine Yeast Lees Valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Union, C. of the E. Council Regulation (EEC). No 337/79 of 5 February 1979 on the Common Organization of the Market in Wine; EEC: Brussels, Belgium, 1979; pp. 1–47. [Google Scholar]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Bibbins, B.; Torrado-Agrasar, A.; Salgado, J.M.; Oliveira, R.P.d.S.; Domínguez, J.M. Potential of Lees from Wine, Beer and Cider Manufacturing as a Source of Economic Nutrients: An Overview. Waste Manag. 2015, 40, 72–81. [Google Scholar] [CrossRef]
- Ye, Z.; Harrison, R.; Cheng, V.; Bekhit, A. Wine Making By-Products. In Valorization of Wine Making By-Products; CRC Press: Boca Raton, FL, USA, 2015; pp. 73–116. [Google Scholar]
- Romero-Díez, R.; Rodrigues, L.; Rodríguez-Rojo, S.; Cocero, M.; Matias, A. Pretreatment Effect on Anthocyanin Extraction Kinetics from Different Wine Lees. In Proceedings of the 13th International Conference Renewable Resources, Wroclaw, Poland, 7–9 June 2017. [Google Scholar]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A Novel Approach for the Valorization of Wine Lees as a Source of Compounds Able to Modify Wine Properties. LWT 2021, 136, 110274. [Google Scholar] [CrossRef]
- Cunniff, P. Official Method 969.33 of AOAC International. In Official Methods of Analysis of AOAC Internationa; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Sancho-Pardo, L.; Bravo, F.I.; Mulero, M.; Muguerza, B.; Arola-Arnal, A. Optimized Extraction by Response Surface Methodology Used for the Characterization and Quantification of Phenolic Compounds in Whole Red Grapes (Vitis Vinifera). Nutrients 2018, 10, 1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Suarez, M.; Muguerza, B.; Bravo, F.I. Long-Term Administration of Protein Hydrolysate from Chicken Feet Induces Antihypertensive Effect and Confers Vasoprotective Pattern in Diet-Induced Hypertensive Rats. J. Funct. Foods 2019, 55, 28–35. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the Degree of Hydrolysis of Food Protein Hydrolysates by Trinitrobenzenesulfonic Acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant Activity In Vitro of the Selenium-Contained Protein from the Se-Enriched Bifidobacterium Animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.; Miguel, M.; Muguerza, B.; Aleixandre, A. Effect of a Cocoa Polyphenol Extract in Spontaneously Hypertensive Rats. Food Funct. 2011, 2, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Hogervorst, J.C.; Miljić, U.; Puškaš, V. Extraction of Bioactive Compounds from Grape Processing By-Products. In Handbook of Grape Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 105–135. [Google Scholar]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an Enzyme Preparation with Industrial Relevance: Automated Nine-Step Purification and Partial Characterization of Eight Enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.A.; Sun, D.W. Ultrasound-Assisted Extraction of Phenolics from Wine Lees: Modeling, Optimization and Stability of Extracts during Storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Barcia, M.T.; Pertuzatti, P.B.; Rodrigues, D.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; Godoy, H.T. Occurrence of Low Molecular Weight Phenolics in Vitis Vinifera Red Grape Cultivars and Their Winemaking By-Products from São Paulo (Brazil). Food Res. Int. 2014, 62, 500–513. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; Luque de Castro, M.D. Microwave-Assisted Extraction of Phenolic Compounds from Wine Lees and Spray-Drying of the Extract. Food Chem. 2011, 124, 1652–1659. [Google Scholar] [CrossRef]
- Senevirathne, M.; Kim, S.-H.; Jeon, Y.-J. Protective Effect of Enzymatic Hydrolysates from Highbush Blueberry (Vaccinium Corymbosum L.) against Hydrogen Peroxide-Induced Oxidative Damage in Chinese Hamster Lung Fibroblast Cell Line. Nutr. Res. Pr. 2010, 4, 183. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell Wall Anthocyanin Adsorption by Different Saccharomyces Strains during the Fermentation of Vitis Vinifera L. Cv Graciano Grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Mena, P.; Ascacio-Valdés, J.A.; Gironés-Vilaplana, A.; Del Rio, D.; Moreno, D.A.; García-Viguera, C. Assessment of Pomegranate Wine Lees as a Valuable Source for the Recovery of (Poly)Phenolic Compounds. Food Chem. 2014, 145, 327–334. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic Characterization of Aging Wine Lees: Correlation with Antioxidant Activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Bei, Q.; Chen, G.; Liu, Y.; Zhang, Y.; Wu, Z. Improving Phenolic Compositions and Bioactivity of Oats by Enzymatic Hydrolysis and Microbial Fermentation. J. Funct. Foods 2018, 47, 512–520. [Google Scholar] [CrossRef]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Rusak, G.; Likić, S.; Berendika, M. Phenolic Composition and Antioxidant Properties of Some Traditionally Used Medicinal Plants Affected by the Extraction Time and Hydrolysis. Phytochem. Anal. 2011, 22, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Griendling, K.K. Reactive Oxygen Species in the Vasculature: Molecular and Cellular Mechanisms. Hypertension 2003, 39, 788–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campia, U.; Tesauro, M.; Cardillo, C. Human Obesity and Endothelium-Dependent Responsiveness. Br. J. Pharmacol. 2012, 165, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Tzakos, A.G.; Naqvi, N.; Comporozos, K.; Pierattelli, R.; Theodorou, V.; Husain, A.; Gerothanassis, I.P. The Molecular Basis for the Selection of Captopril Cis and Trans Conformations by Angiotensin I Converting Enzyme. Bioorganic Med. Chem. Lett. 2006, 16, 5084–5087. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Murphy, T.J.; Alexander, R.W. Molecular Biology of the Renin-Angiotensin System. Circulation 1993, 87, 1816–1828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, Y.M.; Paul, M.; Ganten, D. Lessons from Rat Models of Hypertension: From Goldblatt to Genetic Engineering. Cardiovasc. Res. 1998, 39, 77–88. [Google Scholar] [CrossRef]
- Gao, X.; Xue, Z.; Ma, Q.; Guo, Q.; Xing, L.; Santhanam, R.K.; Zhang, M.; Chen, H. Antioxidant and Antihypertensive Effects of Garlic Protein and Its Hydrolysates and the Related Mechanism. J. Food Biochem. 2020, 44, e13126. [Google Scholar] [CrossRef]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [Green Version]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.-M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher Anthocyanin Intake Is Associated with Lower Arterial Stiffness and Central Blood Pressure in Women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, K.; Charlton, K.E.; Jenner, A.; Roodenrys, S. Acute Reduction in Blood Pressure Following Consumption of Anthocyanin-Rich Cherry Juice May Be Dose-Interval Dependant: A Pilot Cross-over Study. Int. J. Food Sci. Nutr. 2016, 67, 47–52. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Wang, X.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. The Effect of Anthocyanins on Blood Pressure. Medicine 2016, 95, e3380. [Google Scholar] [CrossRef] [PubMed]
- Calfío, C.; Huidobro-Toro, J.P. Potent Vasodilator and Cellular Antioxidant Activity of Endemic Patagonian Calafate Berries (Berberis Microphylla) with Nutraceutical Potential. Molecules 2019, 24, 2700. [Google Scholar] [CrossRef] [Green Version]
- Alañón, M.E.; Castle, S.M.; Serra, G.; Lévèques, A.; Poquet, L.; Actis-Goretta, L.; Spencer, J.P.E. Acute Study of Dose-Dependent Effects of (−)-Epicatechin on Vascular Function in Healthy Male Volunteers: A Randomized Controlled Trial. Clin. Nutr. 2020, 39, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Weber, T.; Skene, S.S.; Ottaviani, J.I.; Crozier, A.; Kelm, M.; Schroeter, H.; Heiss, C. Assessing the Respective Contributions of Dietary Flavanol Monomers and Procyanidins in Mediating Cardiovascular Effects in Humans: Randomized, Controlled, Double-Masked Intervention Trial. Am. J. Clin. Nutr. 2018, 108, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin Improves Vascular Endothelial Function through Promotion of Autophagy in Hypertensive Rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin Reduces Blood Pressure in Hypertensive Subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Jimenez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive Effects of the Flavonoid Quercetin. Pharm. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Kang, N.; Lee, J.-H.; Lee, W.; Ko, J.-Y.; Kim, E.-A.; Kim, J.-S.; Heu, M.-S.; Kim, G.H.; Jeon, Y.-J. Gallic Acid Isolated from Spirogyra Sp. Improves Cardiovascular Disease through a Vasorelaxant and Antihypertensive Effect. Environ. Toxicol. Pharm. 2015, 39, 764–772. [Google Scholar] [CrossRef] [PubMed]
- OIV. Código Prácticas Enológicas; OIV: Paris, France, 2016; Volume 33. [Google Scholar]




| Amino Acids | 0 WL (µg/g) | PWL (µg/g) |
|---|---|---|
| Alanine | 706.3 ± 62.8 | 854.5 ± 70.1 |
| Arginine | 110.5 ± 12.5 | 195.2 ± 19.9 |
| Asparagine | 273.6 ± 59.5 | 417.6 ± 91.0 |
| Aspartate | 307.9 ± 37.4 | 702.9 ± 78.3 |
| Glutamate | 1086.1 ± 265.5 | 1278.4 ± 325.8 |
| Glutamine | N.D | N.D |
| Glycine | 345.5 ± 65.2 | 442.1 ± 68.8 |
| Histidine | 75.6 ± 9.3 | 135.4 ± 7.2 |
| Isoleucine | 358.8 ± 54.7 | 2014.6 ± 256.2 ** |
| Leucine | 435.8 ± 74.4 | 2857.6 ± 445.7 ** |
| Lysine | 143.7 ± 28.2 | 238.4 ± 46.6 |
| Phenylalanine | 64.9 ± 11.7 | 189.9 ± 34.8 |
| Proline | 19,828.7 ± 1230.8 | 28,623.8 ± 1761.1 ** |
| Serine | 517.0 ± 188.6 | 1091.9 ± 348.0 |
| Threonine | 326.2 ± 87.7 | 720.5 ± 181.0 |
| Tryptohan | N.D | 52.9 ± 18.6 * |
| Tyrosine | 46.8 ± 7.2 | 152.8 ± 19.9 |
| Valine | 181.7 ± 29.4 | 1187.0 ± 172.4 * |
| Methionine | 25.0 ± 7.6 | 46.8 ± 13.1 |
| Cystine | N.D | N.D |
| Hydroxiproline | N.D | N.D |
| TOTAL | 24,834.1 | 41,202.3 |
| Compound | R.T. (min) | [M-H]- | Fragment (m/z) | 0 WL (µg/g) | PWL (µg/g) | |
|---|---|---|---|---|---|---|
| Flavanols | ||||||
| 1 | Catechin | 8.17 | 289.0718 | 2681.20 ± 19.20 | 3289.60 ± 20.80 * | |
| 2 | Catechin gallate a | 6.66 | 441.0827 | 289.07209 | 18.00 ± 0.40 | 16.40 ± 0.40 ** |
| 3 | Epicatechin | 9.96 | 289.0718 | 1035.60 ± 6.00 | 1242.00 ± 6.80 * | |
| 4 | (Epi)catechin O-glucoside iso1 b | 6.55 | 451.1246 | 289.0721 | 20.80 ± 0.00 | 22.80 ± 0.00 * |
| 5 | (Epi)catechin O-glucoside iso2 b | 7.41 | 451.1246 | 289.0721 | 10.80 ± 0.00 | 16.40 ± 0.00 * |
| 6 | (Epi)catechin O-glucoside iso3 b | 8.37 | 451.1246 | 289.0721 | 62.80 ± 1.20 | 70.80 ± 1.20 * |
| 7 | Procyanidin dimer B2 | 9.30 | 577.1387 | 289.0733 | 514.40 ± 0.40 | 634.00 ± 0.40 ** |
| 8 | Procyanidin dimer iso1 c | 7.68 | 577.1387 | 289.0733 | 1020.80 ± 6.00 | 1178.00 ± 6.40 * |
| 9 | Procyanidin dimer iso2 c | 7.97 | 577.1387 | 289.0733 | 276.80 ± 2.00 | 334.40 ± 2.00 * |
| 10 | Procyanidin dimer iso3 c | 8.18 | 577.1387 | 289.0733 | 85.20 ± 0.80 | 86.80 ± 0.40 |
| 11 | Procyanidin dimer iso4 c | 8.99 | 577.1387 | 289.0733 | 211.20 ± 0.00 | 262.80 ± 0.00 * |
| 12 | Procyanidin dimer iso5 c | 11.14 | 577.1387 | 289.0733 | 70.40 ± 0.80 | 106.00 ± 1.20 |
| 13 | Procyanidin trimer iso1 c | 5.46 | 865.2016 | 577.1369 | 206.00 ± 4.00 | 251.60 ± 4.40 |
| 14 | Procyanidin trimer iso2 c | 8.67 | 865.2016 | 577.1369 | 184.00 ± 10.40 | 274.00 ± 13.60 * |
| 15 | Procyanidin trimer iso3 c | 8.89 | 865.2016 | 577.1369 | 72.40 ± 0.40 | 84.00 ± 0.40 * |
| 16 | Procyanidin trimer iso4 c | 10.55 | 865.2016 | 577.1369 | 68.80 ± 3.60 | 89.60 ± 0.40 * |
| 17 | Procyanidin trimer iso5 c | 10.71 | 865.2016 | 577.1369 | 177.60 ± 3.60 | 265.60 ± 4.40 * |
| Flavonols | ||||||
| 18 | Quercetin | 17.80 | 301.0372 | 888.40 ± 4.80 | 1954.40 ± 9.20 ** | |
| 19 | Quercetin-3-O-glucoside d | 13.00 | 463.0904 | 301.0361 | 19.20 ± 0.40 | 48.40 ± 1.20 ** |
| 20 | Quercetin-3-O-glucuronide d | 12.95 | 477.0702 | 301.0369 | 115.20 ± 0.80 | 255.20 ± 1.20 ** |
| 21 | Kaempferol d | 20.07 | 285.0405 | 319.60 ± 1.60 | 632.00 ± 2.40 ** | |
| 22 | Kaempferol-3-O-glucuronide d | 14.22 | 461.0763 | 285.0412 | 27.60 ± 0.40 | 66.00 ± 0.80 ** |
| 23 | Isorhamnetin d | 20.31 | 315.0531 | 203.20 ± 2.40 | 481.60 ± 5.20 ** | |
| Phenolic acids | ||||||
| 24 | Gallic acid | 3.13 | 169.0193 | 2734.80 ± 93.60 | 3496.80 ± 106.40 | |
| 25 | Caffeic acid | 8.63 | 179.0401 | 70.80 ± 0.80 | 97.20 ± 1.20 * | |
| 26 | Caffeic acid O-glucoside iso1 e | 7.64 | 341.0878 | 179.0350 | 22.40 ± 0.80 | 23.20 ± 0.80 |
| 27 | Caffeic acid O-glucoside iso2 e | 8.29 | 341.0878 | 179.0350 | 22.40 ± 1.20 | 19.60 ± 0.80 |
| 28 | p-Coumaric acid | 10.65 | 163.0439 | 28.00 ± 0.40 | 117.60 ± 1.20 ** | |
| 29 | 4-Hydroxybenzoic acid | 8.17 | 137.0243 | 58.40 ± 2.00 | 66.40 ± 2.00 | |
| 30 | Ferulic acid | 12.00 | 193.0506 | 13.20 ± 0.40 | 18.80 ± 0.40 * | |
| 31 | Vanillic acid | 8.51 | 167.0350 | 76.40 ± 2.40 | 90.40 ± 2.80 | |
| Stilbenes | ||||||
| 32 | trans-Resveratrol f | 15.73 | 227.0714 | 120.40 ± 0.80 | 164.00 ± 0.80 ** | |
| 33 | Resveratrol iso1 f | 18.00 | 227.0714 | 66.00 ± 0.40 | 152.40 ± 0.80 ** | |
| 34 | Resveratrol O-glucoside iso1 f | 12.44 | 389.1242 | 227.0721 | 30.00 ± 0.40 | 56.40 ± 0.80 ** |
| 35 | Resveratrol O-glucoside iso2 f | 14.92 | 389.1242 | 227.0721 | 88.00 ± 1.60 | 138.00 ± 2.40 ** |
| 36 | Piceatannol f | 2.59 | 243.0663 | 203.0727 | 124.40 ± 2.00 | 136.80 ± 1.60 * |
| 37 | Piceatannol 3-O-glucoside iso1 f | 12.89 | 405.1208 | 243.0670 | 5.60 ± 0.00 | 9.60 ± 0.40 * |
| 38 | Piceatannol 3-O-glucoside iso2 f | 13.15 | 405.1208 | 243.0670 | 2.40 ± 0.00 | 4.80 ± 0.00 * |
| 39 | Viniferin-iso1 f | 19.53 | 453.1344 | 116.9291 | 4.40 ± 0.00 | 6.40 ± 0.00 * |
| 40 | Viniferin-iso2 f | 19.92 | 453.1344 | 116.9291 | 20.80 ± 0.40 | 29.20 ± 0.80 * |
| Anthocyanins | R.T. (min) | [M-H]+ | Fragment (m/z) | 0 WL (µg/g) | PWL (µg/g) | |
|---|---|---|---|---|---|---|
| 1 | Gallocatechin-malvidin-3-glucoside dimer a | 3.58 | 797.2035 | 1.60 ± 0.04 | 4.46 ± 0.11 * | |
| 2 | Malvidin-3-glucoside-(epi)catechin a | 4.84 | 781.1974 | 16.67 ± 0.13 | 19.27 ± 0.14 * | |
| 3 | Delphinidin-3-glucoside b | 5.06 | 465.1028 | 303.0511 | 184.88 ± 1.82 | 573.34 ± 5.64 ** |
| 4 | Cyanidin-3-glucoside b | 5.85 | 449.1078 | 287.0531 | 21.72 ± 0.81 | 48.11 ± 1.79 * |
| 5 | Petunidin-3-glucoside c | 6.47 | 479.1184 | 317.0669 | 195.11 ± 2.26 | 513.73 ± 5.96 ** |
| 6 | Petunidin-3-glucoside-pyruvic acid c | 7.05 | 547.1082 | 385.0547 | 1.61 ± 0.04 | 3.54 ± 0.09 * |
| 7 | Peonidin-3-glucoside c | 7.14 | 463.1235 | 301.0717 | 151.78 ± 2.50 | 312.22 ± 5.14 * |
| 8 | Malvidin-3-glucoside a | 7.48 | 493.1341 | 331.0843 | 1780.76 ± 20.01 | 3334.75 ± 37.47 * |
| 9 | Peonidin-3-glucoside-pyruvic acid c | 7.81 | 531.1133 | 369.0607 | 0.93 ± 0.03 | 1.90 ± 0.06 * |
| 10 | Delphinidin-(6-acetyl)-3-glucoside b | 7.87 | 507.1133 | 303.0496 | 47.82 ± 0.90 | 151.91 ± 2.85 * |
| 11 | Visitin A (malvidin-3-glucoside-pyruvic acid) a | 8.11 | 561.1239 | 399.0730 | 14.80 ± 0.13 | 31.02 ± 0.27 ** |
| 12 | Visitin B (malvidin-3-glucoside-acetaldehyde) a | 8.32 | 517.1341 | 355.0826 | 24.08 ± 0.59 | 70.03 ± 1.73 * |
| 13 | Malvidin-3-glucoside-ethyl-(epi)catechin a | 8.40 | 809.2287 | 4.62 ± 0.03 | 8.91 ± 0.05 * | |
| 14 | Cyanidin-(6-acetyl)-3-glucoside b | 8.45 | 491.1184 | 491.1189 | 15.21 ± 0.23 | 33.29 ± 0.50 * |
| 15 | Acetylvisitin A a | 8.50 | 603.1344 | 399.0718 | 13.66 ± 0.44 | 19.65 ± 0.63 * |
| 16 | Malvidin-3-glucoside-ethyl-(epi)catechin a | 8.57 | 809.2287 | 17.54 ± 0.24 | 31.82 ± 0.43 * | |
| 17 | Petunidin-(6-acetyl)-3-glucoside c | 8.66 | 521.1378 | 317.0667 | 55.81 ± 1.87 | 150.18 ± 5.04 ** |
| 18 | Malvidin-3-glucoside-ethyl-(epi)catechin a | 8.75 | 809.2287 | 22.92 ± 0.73 | 42.10 ± 1.34 ** | |
| 19 | Acetylvisitin B a | 8.77 | 559.1446 | 355.0813 | 14.50 ± 0.44 | 36.86 ± 1.12 * |
| 20 | Peonidin-(6-acetyl)-3-glucoside c | 9.08 | 505.1341 | 301.0714 | 55.47 ± 1.24 | 124.75 ± 2.80 * |
| 21 | Delphinidin-(6-coumaroyl)-3-glucoside b | 9.08 | 611.1395 | 303.0508 | 14.72 ± 0.27 | 48.48 ± 0.89 * |
| 22 | Malvidin-(6-acetyl)-3-glucoside a | 9.13 | 535.1446 | 331.0836 | 727.35 ± 0.84 | 1503.53 ± 1.74 ** |
| 23 | Coumaroylvisitin A a | 9.29 | 707.1607 | 399.0718 | 2.88 ± 0.07 | 6.18 ± 0.15 * |
| 24 | Malvidin-(6-caffeoyl)-3-glucoside a | 9.41 | 655.1657 | 331.0808 | 5.99 ± 0.27 | 13.98 ± 0.63 * |
| 25 | Cyanidin-(6-coumaroyl)-3-glucoside b | 9.42 | 595.1446 | 287.0560 | 5.01 ± 0.16 | 13.77 ± 0.43 * |
| 26 | Catechin-ethyl-malvidin-3-acetylglucoside dimer a | 9.43 | 851.2511 | 10.30 ± 0.31 | 19.05 ± 0.56 ** | |
| 27 | Petunidin-(6-coumaroyl)-3-glucoside c | 9.52 | 625.1552 | 317.0662 | 21.11 ± 0.36 | 63.19 ± 1.08 * |
| 28 | Pinotin A (malvidin-3-glucoside-vinylcatechol) a | 9.53 | 625.1552 | 463.0998 | 23.80 ± 0.51 | 71.25 ± 1.52 * |
| 29 | Malvidin-glucoside-vinyl-catechin a | 9.56 | 805.1974 | 1.46 ± 0.03 | 4.23 ± 0.09 * | |
| 30 | Coumaroylvisitin B a | 9.58 | 663.1708 | 355.0822 | 8.53 ± 0.28 | 23.20 ± 0.76 * |
| 31 | Malvidin-3-glucoside-vinylguaiacol a | 9.63 | 639.1708 | 331.0823 | 10.07 ± 0.20 | 27.49 ± 0.54 * |
| 32 | Catechin-ethyl-malvidin-3-coumaroylglucoside dimer a | 9.70 | 955.2785 | 5.95 ± 0.11 | 13.77 ± 0.25 * | |
| 33 | Catechin-ethyl-malvidin-3-acetylglucoside dimer a | 9.81 | 851.2511 | 1.93 ± 0.06 | 4.27 ± 0.14 * | |
| 34 | Peonidin-(6-coumaroyl)-3-glucoside c | 9.87 | 609.1603 | 301.0716 | 38.72 ± 1.08 | 85.15 ± 2.37 * |
| 35 | Malvidin-(6-coumaroyl)-3-glucoside a | 9.92 | 639.1708 | 331.0823 | 274.63 ± 0.60 | 768.26 ± 1.69 ** |
| 36 | Malvidin-glucoside-vinyl-catechin a | 9.99 | 805.1974 | 1.58 ± 0.02 | 3.74 ± 0.05 ** | |
| 37 | Acetyl-pinotin A a | 10.19 | 667.1657 | 0.08 ± 0.00 | 0.26 ± 0.00 * | |
| 38 | Malvidin 3-O-glucoside 4-vinylphenol (Pigment A) a | 10.22 | 609.1603 | 447.1079 | 7.40 ± 0.08 | 19.53 ± 0.21 ** |
| 39 | Catechin-ethyl-malvidin-3-coumaroylglucoside dimer a | 10.33 | 955.2785 | 1.14 ± 0.01 | 3.06 ± 0.02 * | |
| 40 | Malvidin acetyl 3-O-glucoside 4-vinylphenol (Acetyl-pigment A) a | 10.50 | 651.1708 | 447.1076 | 4.46 ± 0.17 | 10.42 ± 0.39 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Fernández-Sobrino, R.; Margalef, M.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats. Antioxidants 2021, 10, 517. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10040517
López-Fernández-Sobrino R, Margalef M, Torres-Fuentes C, Ávila-Román J, Aragonès G, Muguerza B, Bravo FI. Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats. Antioxidants. 2021; 10(4):517. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10040517
Chicago/Turabian StyleLópez-Fernández-Sobrino, Raúl, Maria Margalef, Cristina Torres-Fuentes, Javier Ávila-Román, Gerard Aragonès, Begoña Muguerza, and Francisca Isabel Bravo. 2021. "Enzyme-Assisted Extraction to Obtain Phenolic-Enriched Wine Lees with Enhanced Bioactivity in Hypertensive Rats" Antioxidants 10, no. 4: 517. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox10040517