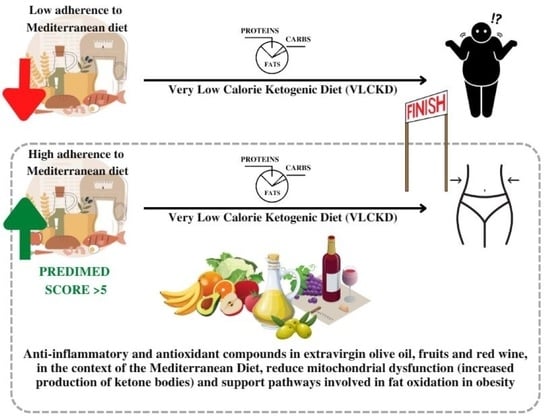
The Antioxidant Potential of the Mediterranean Diet as a Predictor of Weight Loss after a Very Low-Calorie Ketogenic Diet (VLCKD) in Women with Overweight and Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Setting
- a)
- Inclusion criteria: 18 years of age or older; overweight or obesity in accordance with a BMI of ≥ 27.0 kg/m2; naïve subjects (no previous treatment with anti-obesity drugs or bariatric surgery);
- b)
- Exclusion criteria: type 1 diabetes mellitus or T2DM on insulin therapy or latent autoimmune diabetes in adults; renal, hepatic, cardiac (NYHA III-IV), or respiratory insufficiency; unstable angina; stroke or myocardial infarction in the last 12 months; cardiac arrhythmias; psychiatric and eating disorders; alcohol and drug addiction; active and/or severe infections; rare metabolic diseases; pregnancy; lactation.
2.2. Anthropometric Measurements and Physical Activity
2.3. Bioelectrical Impedance Analysis
2.4. Assessment of the Adherence to MD
2.5. VLCKD Intervention
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alharbi, K.K.; Al-Sheikh, Y.A.; Alsaadi, M.M.; Mani, B.; Udayaraja, G.; Kohailan, M.; Khan, I.A. Screening for obesity in the offspring of first-cousin consanguineous couples: A Phase-I study in Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 242–246. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Obesity and Overweight—Key Facts. 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 December 2022).
- WHO. WHO Regional Obesity Report. Available online: https://www.who.int/europe/publications/i/item/9789289057738 (accessed on 12 December 2022).
- Nani, A.; Murtaza, B.; Khan, A.S.; Khan, N.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. Energy Balance and Obesity. Circulation 2012, 126, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Pugliese, G.; Muscogiuri, G.; Laudisio, D.; Colao, A.; Savastano, S. New-generation anti-obesity drugs: Naltrexone/bupropion and liraglutide. An update for endocrinologists and nutritionists. Minerva Endocrinol. 2020, 45, 127–137. [Google Scholar] [CrossRef]
- Cataldi, M.; Muscogiuri, G.; Savastano, S.; Barrea, L.; Guida, B.; Taglialatela, M.; Colao, A. Gender-related issues in the pharmacology of new anti-obesity drugs. Obes. Rev. 2019, 20, 375–384. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalón, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef]
- Gantenbein, K.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Laudisio, D.; Pugliese, G.; Salzano, C.; Savastano, S.; Colao, A. The management of very low-calorie ketogenic diet in obesity outpatient clinic: A practical guide. J. Transl. Med. 2019, 17, 356. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L.; Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European Guidelines for Obesity Management in Adults with a Very Low-Calorie Ketogenic Diet: A Systematic Review and Meta-Analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Castellana, M.; Conte, E.; Cignarelli, A.; Perrini, S.; Giustina, A.; Giovanella, L.; Giorgino, F.; Trimboli, P. Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 5–16. [Google Scholar] [CrossRef]
- Tragni, E.; Vigna, L.; Ruscica, M.; Macchi, C.; Casula, M.; Santelia, A.; Catapano, A.; Magni, P. Reduction of Cardio-Metabolic Risk and Body Weight through a Multiphasic Very-Low Calorie Ketogenic Diet Program in Women with Overweight/Obesity: A Study in a Real-World Setting. Nutrients 2021, 13, 1804. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boison, D. New insights into the mechanisms of the ketogenic diet. Curr. Opin. Neurol. 2017, 30, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Alshammary, A.F.; Khan, I.A. Screening of Obese Offspring of First-Cousin Consanguineous Subjects for the Angiotensin-Converting Enzyme Gene with a 287-bp Alu Sequence. J. Obes. Metab. Syndr. 2021, 30, 63–71. [Google Scholar] [CrossRef]
- Nishida, C.; Ko, G.T.; Kumanyika, S. Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist–Hip Ratio. Eur. J. Clin. Nutr. 2009, 64, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Pugliese, G.; Savastano, S.; Colao, A.; on behalf of the Opera Prevention Project. Chronotype and Adherence to the Mediterranean Diet in Obesity: Results from the Opera Prevention Project. Nutrients 2020, 12, 1354. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Barrea, L.; Macchia, P.E.; Di Somma, C.; Napolitano, M.; Balato, A.; Falco, A.; Savanelli, M.C.; Balato, N.; Colao, A.; Savastano, S. Bioelectrical phase angle and psoriasis: A novel association with psoriasis severity, quality of life and metabolic syndrome. J. Transl. Med. 2016, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrea, L.; Tarantino, G.; Di Somma, C.; Muscogiuri, G.; Macchia, P.E.; Falco, A.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxid. Med. Cell. Longev. 2017, 2017, 6101254. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Muscogiuri, G.; Aprano, S.; Vetrani, C.; de Alteriis, G.; Varcamonti, L.; Verde, L.; Colao, A.; Savastano, S. Phase angle as an easy diagnostic tool for the nutritionist in the evaluation of inflammatory changes during the active stage of a very low-calorie ketogenic diet. Int. J. Obes. 2022, 46, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Boghossian, N.S.; Yeung, E.H.; Mumford, S.L.; Zhang, C.; Gaskins, A.J.; Wactawski-Wende, J.; Schisterman, E.F.; for the BioCycle Study Group. Adherence to the Mediterranean diet and body fat distribution in reproductive aged women. Eur. J. Clin. Nutr. 2013, 67, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Papadaki, A.; Nolen-Doerr, E.; Mantzoros, C.S. The Effect of the Mediterranean Diet on Metabolic Health: A Systematic Review and Meta-Analysis of Controlled Trials in Adults. Nutrients 2020, 12, 3342. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Salzano, C.; Pugliese, G.; De Alteriis, G.; Colao, A.; Savastano, S. Phase Angle: A Possible Biomarker to Quantify Inflammation in Subjects with Obesity and 25(OH)D Deficiency. Nutrients 2019, 11, 1747. [Google Scholar] [CrossRef] [Green Version]
- Barrea, L.; Caprio, M.; Camajani, E.; Verde, L.; Elce, A.; Frias-Toral, E.; Ceriani, F.; Cucalón, G.; Garcia-Velasquez, E.; El Ghoch, M.; et al. Clinical and nutritional management of very-low-calorie ketogenic diet (VLCKD) in patients with psoriasis and obesity: A practical guide for the nutritionist. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Barrea, L.; Caprio, M.; Watanabe, M.; Cammarata, G.; Feraco, A.; Muscogiuri, G.; Verde, L.; Colao, A.; Savastano, S. Could very low-calorie ketogenic diets turn off low grade inflammation in obesity? Emerging evidence. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Rondanelli, M.; Perna, S.; Ilyas, Z.; Peroni, G.; Bazire, P.; Sajuox, I.; Maugeri, R.; Nichetti, M.; Gasparri, C. Effect of very low-calorie ketogenic diet in combination with omega-3 on inflammation, satiety hormones, body composition, and metabolic markers. A pilot study in class I obese subjects. Endocrine 2022, 75, 129–136. [Google Scholar] [CrossRef]
- Vidali, S.; Aminzadeh, S.; Lambert, B.; Rutherford, T.; Sperl, W.; Kofler, B.; Feichtinger, R.G. Mitochondria: The ketogenic diet—A metabolism-based therapy. Int. J. Biochem. Cell Biol. 2015, 63, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [Green Version]
- de Mello, A.H.; Costa, A.B.; Engel, J.D.G.; Rezin, G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018, 192, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; Britto, J.S.J.; Galerati, I.; Gómez-Ambrosi, J.; Frühbeck, G.; Portincasa, P. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients 2022, 14, 3112. [Google Scholar] [CrossRef]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free. Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-Z.; Li, L.; Espe, M.; Lu, K.-L.; Rahimnejad, S. Hydroxytyrosol Attenuates Hepatic Fat Accumulation via Activating Mitochondrial Biogenesis and Autophagy through the AMPK Pathway. J. Agric. Food Chem. 2020, 68, 9377–9386. [Google Scholar] [CrossRef]
- Gueguen, N.; Desquiret-Dumas, V.; Leman, G.; Chupin, S.; Baron, S.; Nivet-Antoine, V.; Vessières, E.; Ayer, A.; Henrion, D.; Lenaers, G.; et al. Resveratrol Directly Binds to Mitochondrial Complex I and Increases Oxidative Stress in Brain Mitochondria of Aged Mice. PLoS ONE 2015, 10, e0144290. [Google Scholar] [CrossRef] [Green Version]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, Q.; Suo, Y.; Tan, X.; Yuan, T.; Liu, Z.; Liu, X. Lycopene ameliorates systemic inflammation-induced synaptic dysfunction via improving insulin resistance and mitochondrial dysfunction in the liver–brain axis. Food Funct. 2019, 10, 2125–2137. [Google Scholar] [CrossRef]
- Keshtzar, E.; Khodayar, M.; Javadipour, M.; Ghaffari, M.; Bolduc, D.; Rezaei, M. Ellagic acid protects against arsenic toxicity in isolated rat mitochondria possibly through the maintaining of complex II. Hum. Exp. Toxicol. 2015, 35, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, L.; Wang, Y.; Zhang, G.; Cheng, Y.; Feng, Z.; Bai, X.; Liu, J. High ratio of ω-3/ω-6 polyunsaturated fatty acids targets mTORC1 to prevent high-fat diet-induced metabolic syndrome and mitochondrial dysfunction in mice. J. Nutr. Biochem. 2020, 79, 108330. [Google Scholar] [CrossRef]
- Mateș, L.; Popa, D.-S.; Rusu, M.E.; Fizeșan, I.; Leucuța, D. Walnut Intake Interventions Targeting Biomarkers of Metabolic Syndrome and Inflammation in Middle-Aged and Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Antioxidants 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Guo, B.; Xiao, X.; Yin, J.; Wang, Z.; Jiang, X.; Li, J.; Long, L.; Zhou, J.; Zhang, N.; et al. Healthy dietary patterns and metabolic dysfunction-associated fatty liver disease in less-developed ethnic minority regions: A large cross-sectional study. BMC Public Health 2022, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Castro-Barquero, S.; Lamuela-Raventós, R.M.; Doménech, M.; Estruch, R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients 2018, 10, 1523. [Google Scholar] [CrossRef] [Green Version]
- Knol, M.J.; Le Cessie, S.; Algra, A.; Vandenbroucke, J.P.; Groenwold, R.H. Overestimation of risk ratios by odds ratios in trials and cohort studies: Alternatives to logistic regression. Can. Med. Assoc. J. 2011, 184, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Fakhrawi, D.H.; Beeson, L.; Libanati, C.; Feleke, D.; Kim, H.; Quansah, A.; Darnell, A.; Lammi-Keefe, C.J.; Cordero-MacIntyre, Z. Comparison of Body Composition by Bioelectrical Impedance and Dual-Energy X-Ray Absorptiometry in Overweight/Obese Postmenopausal Women. J. Clin. Densitom. 2009, 12, 238–244. [Google Scholar] [CrossRef]
- Thomson, R.; Brinkworth, G.D.; Buckley, J.D.; Noakes, M.; Clifton, P.M. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin. Nutr. 2007, 26, 771–777. [Google Scholar] [CrossRef]



| Parameters N = 318 | Baseline | End of Active Phase of VLCKD | ∆% | * p-Value |
|---|---|---|---|---|
| Weight (kg) | 95.15 ± 15.89 | 88.14 ± 14.91 | −7.35 ± 2.72 | <0.001 |
| BMI (kg/m2) | 35.75 ± 5.18 | 33.11 ± 4.88 | −7.36 ± 2.73 | <0.001 |
| Normal weight (n, %) | - | 7, 2.2 | 2.2 | χ2 = 5.20, p = 0.023 |
| Overweight (n, %) | 47, 14.8 | 96, 30.2 | 15.4 | χ2 = 20.79, p < 0.001 |
| Grade I obesity (n, %) | 104, 32.7 | 105, 33.0 | 0.3 | χ2 = 0.00, p = 1.000 |
| Grade II obesity (n, %) | 100, 31.4 | 81, 25.5 | −5.9 | χ2 = 2.50, p = 0.114 |
| Grade III obesity (n, %) | 67, 21.1 | 29, 9.1 | −12.0 | χ2 = 16.80, p < 0.001 |
| WC (cm) | 104.84 ± 14.58 | 98.39 ± 13.63 | −5.96 ± 4.56 | <0.001 |
| BIA | ||||
| R (Ω) | 477.36 ± 69.94 | 483.81 ± 67.29 | 1.76 ± 8.35 | 0.003 |
| Xc (Ω) | 47.64 ± 9.72 | 51.17 ± 9.52 | 8.75 ± 15.18 | <0.001 |
| FM (kg) | 40.98 ± 12.64 | 34.83 ± 11.52 | −15.06 ± 8.85 | <0.001 |
| FM (%) | 42.24 ± 6.59 | 38.66 ± 6.94 | −8.53 ± 7.78 | <0.001 |
| FFM (kg) | 54.16 ± 5.79 | 53.24 ± 6.02 | −1.65 ± 4.76 | <0.001 |
| FFM (%) | 57.76 ± 6.59 | 61.33 ± 6.94 | 6.33 ± 5.60 | <0.001 |
| PhA (°) | 5.71 ± 0.86 | 6.04 ± 0.81 | 6.78 ± 11.77 | <0.001 |
| Parameters N = 318 | Low Adherence to MD 117, 36.8% | Average Adherence to MD 172, 54.1% | High Adherence to MD 29, 9.1% | p-Value |
|---|---|---|---|---|
| Baseline parameters | ||||
| Age (years) | 39.46 ± 14.41 | 37.84 ± 14.05 | 42.24 ± 15.88 | 0.264 |
| Weight (kg) | 100.90 ± 16.97 a,b | 93.28 ± 14.19 a | 83.06 ± 11.22 | <0.001 |
| BMI (kg/m2) | 38.13 ± 5.04 a,b | 34.90 ± 4.71 a | 31.23 ± 3.66 | <0.001 |
| WC (cm) | 110.68 ± 13.98 a,b | 102.29 ± 13.89 | 95.51 ± 12.26 | <0.001 |
| R (Ω) | 469.29 ± 64.06 | 481.87 ± 76.10 | 483.21 ± 50.88 | 0.291 |
| Xc (Ω) | 46.01 ± 9.48 | 48.26 ± 10.19 | 50.51 ± 6.45 | 0.038 |
| FM (kg) | 46.00 ± 13.65 a,b | 39.36 ± 11.00 a | 30.32 ± 7.62 | <0.001 |
| FM (%) | 44.81 ± 6.16 a,b | 41.52 ± 6.26 a | 36.10 ± 50.08 | <0.001 |
| FFM (kg) | 54.89 ± 5.83 | 53.90 ± 5.79 | 52.73 ± 5.47 | 0.136 |
| FFM (%) | 55.19 ± 6.16 a,b | 58.48 ± 6.26 a | 63.90 ± 5.08 | <0.001 |
| PhA (°) | 5.60 ± 0.88 | 5.73 ± 0.86 | 5.97 ± 0.61 | 0.091 |
| Parameters at the end of active phase of VLCKD | ||||
| Weight (kg) | 94.65 ± 15.58 a,b | 85.81 ± 13.03 a | 75.76 ± 10.30 | <0.001 |
| Δ% weight | −6.89 ± 2.76 | −7.60 ± 2.71 | −7.75 ± 2.41 | 0.065 |
| BMI (kg/m2) | 35.78 ± 4.61 a,b | 32.09 ± 4.27 a | 28.49 ± 3.46 | <0.001 |
| Δ% BMI | −6.11 ± 2.30 a,b | −7.99 ± 2.57 | −8.72 ± 3.34 | <0.001 |
| WC (cm) | 104.22 ± 12.79 a,b | 95.79 ± 12.99 | 90.24 ± 12.02 | <0.001 |
| Δ% WC | −5.61 ± 4.26 | −6.17 ± 4.53 | −6.33 ± 5.96 | 0.539 |
| R (Ω) | 488.74 ± 66.64 | 481.41 ± 69.66 | 478.13 ± 55.38 | 0.592 |
| Δ% R | 4.54 ± 9.58 a,b | 0.34 ± 7.37 | −1.02 ± 4.99 | <0.001 |
| Xc (Ω) | 50.25 ± 10.33 | 51.71 ± 9.39 | 51.66 ± 6.35 | 0.428 |
| Δ% Xc | 10.41 ± 16.98 | 8.57 ± 13.91 | 3.15 ± 13.75 | 0.068 |
| FM (kg) | 41.06 ± 11.85 a,b | 32.53 ± 9.46 a | 23.33 ± 6.63 | <0.001 |
| Δ% FM | −10.29 ± 6.73 a,b | −17.07 ± 8.14 | −23.17 ± 10.15 | <0.001 |
| FM (%) | 42.70 ± 5.80 a,b | 37.28 ± 6.05 a | 30.51 ± 5.82 | <0.001 |
| Δ% FM | −4.49 ± 6.06 a,b | −10.06 ± 7.23 a | −15.78 ± 8.76 | <0.001 |
| FFM (kg) | 53.59 ± 6.00 | 53.29 ± 5.93 | 51.47 ± 6.54 | 0.236 |
| Δ% FFM | −2.34 ± 4.61 b | −1.07 ± 4.07 | −2.24 ± 7.98 | 0.065 |
| FFM (%) | 57.30 ± 5.80 a,b | 62.69 ± 6.07 a | 69.49 ± 5.82 | <0.001 |
| Δ% FFM | −4.49 ± 6.06 a,b | −10.06 ± 7.23 | −15.78 ± 8.76 | <0.001 |
| PhA (°) | 5.86 ± 0.86 b | 6.15 ± 0.81 | 6.17 ± 0.50 | 0.010 |
| Δ% PhA | 5.50 ± 11.67 | 8.24 ± 11.77 | 4.08 ± 11.58 | 0.063 |
| Parameters N = 318 | Low Adherence to MD 117, 36.8% | Average Adherence to MD 172, 54.1% | High Adherence to MD 29, 9.1% | p-Value |
|---|---|---|---|---|
| Baseline parameters | ||||
| Physical activity | ||||
| Yes (n, %) | 35, 29.9 | 52, 30.2 | 15, 51.7 | χ2 = 5.56 p = 0.059 |
| No (n, %) | 82, 70.1 | 120, 69.8 | 14, 18.3 | |
| BMI categories | ||||
| Normal weight (n, %) | 0, 0 | 0, 0 | 0, 0 | - |
| Overweight (n, %) | 1, 0.9 | 32, 18.6 | 14, 48.3 | χ2 = 45.84, p < 0.001 |
| Grade I obesity (n, %) | 37, 31.6 | 57, 33.1 | 10, 34.5 | χ2 = 0.12, p = 0.943 |
| Grade II obesity (n, %) | 36, 30.8 | 60, 34.9 | 4, 13.8 | χ2 = 5.16, p = 0.076 |
| Grade III obesity (n, %) | 43, 36.8 | 23, 13.4 | 1, 3.4 | χ2 = 28.85, p < 0.001 |
| Parameters at the end of active phase of VLCKD | ||||
| Physical activity | ||||
| Yes (n, %) | 35, 29.9 | 52, 30.2 | 15, 51.7 | χ2 = 5.56 p = 0.059 |
| No (n, %) | 82, 70.1 | 120, 69.8 | 14, 18.3 | |
| BMI categories | ||||
| Normal weight (n, %) | 0, 0% | 3, 1.7 | 4, 13.8 | χ2 = 20.90, p < 0.001 |
| Overweight (n, %) | 16, 13.7 | 61, 35.5 | 19, 65.5 | χ2 = 34.59, p < 0.001 |
| Grade I obesity (n, %) | 37, 31.6 | 65, 37.8 | 3, 10.3 | χ2 = 8.62, p = 0.014 |
| Grade II obesity (n, %) | 42, 35.9 | 36, 20.9 | 3, 10.3 | χ2 = 12.06, p = 0.002 |
| Grade III obesity (n, %) | 22, 18.8 | 7, 4.1 | 0, 0 | χ2 = 21.44, p < 0.001 |
| Questions of PREDIMED questionnaire N = 318 | ||
|---|---|---|
| n | % | |
| Use of EVOO as main culinary lipid | 268 | 84.3 |
| EVOO > 4 tablespoons | 176 | 55.3 |
| Vegetables ≥ 2 servings/day | 139 | 43.7 |
| Fruits ≥ 3 servings/day | 128 | 40.3 |
| Red/processed meats < 1/day | 163 | 51.3 |
| Butter, cream, margarine < 1/day | 150 | 47.2 |
| Soda drinks < 1/day | 149 | 46.9 |
| Wine glasses ≥ 7/week | 64 | 20.1 |
| Legumes ≥ 3/week | 142 | 44.7 |
| Fish/seafood ≥ 3/week | 109 | 34.3 |
| Commercial sweets and confectionery ≤ 2/week | 118 | 37.1 |
| Tree nuts ≥ 3/week | 126 | 39.6 |
| Poultry more than red meats | 145 | 45.6 |
| Use of sofrito sauce ≥ 2/week | 151 | 47.5 |
| Questions of PREDIMED Questionnaire n = 318 | Below the Median n = 159 | Above the Median n = 159 | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | χ2 | * p-Value | |
| Use of EVOO oil as main culinary lipid | 115 | 72.3 | 153 | 96.2 | 32.49 | <0.001 |
| EVOO > 4 tablespoons | 79 | 49.7 | 97 | 61.0 | 3.68 | 0.055 |
| Vegetables ≥ 2 servings/day | 46 | 28.9 | 93 | 58.5 | 27.04 | <0.001 |
| Fruits ≥ 3 servings/day | 31 | 19.5 | 97 | 61.0 | 55.24 | <0.001 |
| Red/processed meats < 1/day | 66 | 41.5 | 97 | 61.0 | 11.34 | 0.001 |
| Butter, cream, margarine < 1/day | 62 | 39.0 | 88 | 55.3 | 7.89 | 0.005 |
| Soda drinks < 1/day | 64 | 40.3 | 85 | 53.5 | 5.05 | 0.025 |
| Wine glasses ≥ 7/week | 16 | 10.1 | 48 | 30.2 | 18.80 | <0.001 |
| Legumes ≥ 3/week | 63 | 39.6 | 79 | 49.7 | 2.86 | 0.091 |
| Fish/seafood ≥ 3/week | 43 | 27.0 | 66 | 41.5 | 6.76 | 0.009 |
| Commercial sweets and confectionery ≤ 2/week | 42 | 26.4 | 76 | 47.8 | 14.67 | 0.001 |
| Tree nuts ≥ 3/week | 54 | 34.0 | 72 | 45.3 | 3.80 | 0.049 |
| Poultry more than red meats | 60 | 37.7 | 85 | 53.5 | 7.30 | 0.007 |
| Use of sofrito sauce ≥ 2/week | 66 | 41.5 | 85 | 53.5 | 4.09 | 0.053 |
| Low adherence to MD | 103 | 64.8 | 14 | 8.8 | 104.7 | <0.001 |
| Average adherence to MD | 50 | 31.4 | 122 | 76.7 | 63.84 | <0.001 |
| High adherence to MD | 6 | 3.8 | 23 | 14.5 | 9.71 | 0.002 |
| Parameters | PREDIMED Score N = 318 | |||
|---|---|---|---|---|
| Simple Correlation | After Adjusted for Confounding Variables | |||
| r | p-Value | r | p-Value | |
| Δ% weight | −0.149 | 0.008 | −0.154 | 0.006 |
| Δ% BMI | −0.377 | <0.001 | −0.409 | <0.001 |
| Δ% WC | −0.094 | 0.093 | −0.139 | 0.014 |
| Δ% R | −0.349 | <0.001 | −0.380 | <0.001 |
| Δ% Xc | −0.179 | 0.001 | −0.107 | 0.050 |
| Δ% FM | −0.556 | <0.001 | −0.551 | <0.001 |
| Δ% FFM | 0.188 | 0.001 | 0.528 | <0.001 |
| Δ% PhA | 0.044 | 0.430 | 0.182 | 0.001 |
| Δ% FM (n = 318) | ||||
|---|---|---|---|---|
| Questions | OR | p-Value | 95% IC | R2 |
| Use of EVOO as main culinary lipid | 0.85 | <0.001 | 0.81–0.89 | 0.160 |
| EVOO > 4 tablespoons | 0.96 | 0.002 | 0.94–0.99 | 0.030 |
| Vegetables ≥ 2 servings/day | 0.92 | <0.001 | 0.89–0.95 | 0.112 |
| Fruits ≥ 3 servings/day | 0.91 | <0.001 | 0.88–0.94 | 0.120 |
| Red/processed meats < 1/day | 0.96 | 0.004 | 0.94–0.99 | 0.026 |
| Butter, cream, margarine < 1/day | 0.97 | 0.024 | 0.94–1.00 | 0.016 |
| Soda drinks < 1/day | 0.96 | 0.003 | 0.93–0.98 | 0.029 |
| Wine glasses ≥ 7/week | 0.92 | <0.001 | 0.89–0.95 | 0.074 |
| Legumes ≥ 3/week | 0.97 | 0.012 | 0.94–0.99 | 0.020 |
| Fish/seafood ≥ 3/week | 0.94 | <0.001 | 0.91–0.97 | 0.059 |
| Commercial sweets and confectionery ≤ 2/week | 0.95 | <0.001 | 0.93–0.98 | 0.044 |
| Tree nuts ≥ 3/week | 0.98 | 0.050 | 0.95–1.00 | 0.011 |
| Poultry more than red meats | 0.99 | 0.276 | 0.96–1.01 | 0.004 |
| Use of sofrito sauce ≥ 2/week | 0.98 | 0.195 | 0.96–1.00 | 0.005 |
| Low adherence to MD | 0.88 | <0.001 | 0.85–0.91 | 0.181 |
| Average adherence to MD | 0.94 | <0.001 | 0.92–0.97 | 0.057 |
| High adherence to MD | 0.89 | <0.001 | 0.85–0.94 | 0.078 |
| Parameters | Multiple Regression Analysis n = 318 | |||
|---|---|---|---|---|
| R2 | β | t | * p-Value | |
| Δ% FM | 0.307 | −0.556 | −11.91 | <0.001 |
| Parameters | Multiple Regression analysis N = 318 | |||
|---|---|---|---|---|
| R2 | β | t | * p-Value | |
| PREDIMED score | 0.307 | −0.556 | −11.91 | <0.001 |
| Fruits ≥ 3 servings/day | 0.325 | −0.129 | −2.60 | 0.010 |
| Use of EVOO as main culinary lipid | 0.339 | −0.177 | −3.37 | 0.001 |
| Wine glasses ≥ 7/week | 0.356 | −0.147 | −3.05 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verde, L.; Dalamaga, M.; Capó, X.; Annunziata, G.; Hassapidou, M.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G.; Barrea, L. The Antioxidant Potential of the Mediterranean Diet as a Predictor of Weight Loss after a Very Low-Calorie Ketogenic Diet (VLCKD) in Women with Overweight and Obesity. Antioxidants 2023, 12, 18. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12010018
Verde L, Dalamaga M, Capó X, Annunziata G, Hassapidou M, Docimo A, Savastano S, Colao A, Muscogiuri G, Barrea L. The Antioxidant Potential of the Mediterranean Diet as a Predictor of Weight Loss after a Very Low-Calorie Ketogenic Diet (VLCKD) in Women with Overweight and Obesity. Antioxidants. 2023; 12(1):18. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12010018
Chicago/Turabian StyleVerde, Ludovica, Maria Dalamaga, Xavier Capó, Giuseppe Annunziata, Maria Hassapidou, Annamaria Docimo, Silvia Savastano, Annamaria Colao, Giovanna Muscogiuri, and Luigi Barrea. 2023. "The Antioxidant Potential of the Mediterranean Diet as a Predictor of Weight Loss after a Very Low-Calorie Ketogenic Diet (VLCKD) in Women with Overweight and Obesity" Antioxidants 12, no. 1: 18. https://0-doi-org.brum.beds.ac.uk/10.3390/antiox12010018