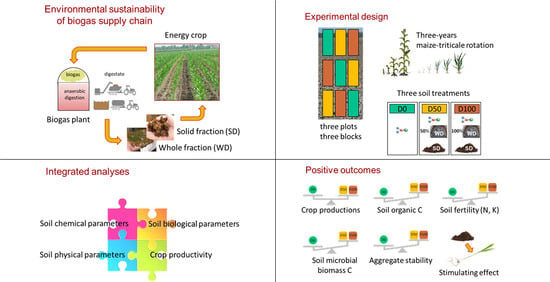
1. Introduction
Interest in biogas production has grown significantly in the past two decades, following the need to reduce fossil fuel consumption in favour of renewable energy sources. To encourage biogas market penetration, EU policy issued financial incentives [
1] which have led to a significant increase in the number of biogas plants. More than 18,000 biogas plants were registered by October 2020 [
2] with an overall installed electric capacity (IEC) of 13,520 MW estimated at the end of 2019 [
3]. Currently, in Europe, Italy and Germany rank first in terms of the number of active biogas plants, with most Italian plants (1900 units with an IEC of around 1000 MW) located in the Po Valley and other northern regions [
4,
5].
Biogas production from anaerobic digestion mainly relies on four types of biomass sources: (i) biomass wastes from farms (animal slurries and crop residues) and households (municipal solid waste and food waste); (ii) agro-industrial by-products; (iii) sewage sludges; (iv) biomass from dedicated energy crops [
6].
The energy derived from anaerobic digestion is considered to be almost “carbon-neutral” and to bring environmental and social benefits, contributing to a reduction of greenhouse gas emissions (allowed by replacement of fossil fuels) and organic wastes [
7], and supporting rural development and new employment opportunities [
8]. Against these benefits, biogas production from energy crops generates several issues and conflicts that are under the political attention on a world scale, since the shift of farmland to non-food systems creates doubts concerning the security of food supply and the environmental impact of energy crops cultivation [
9]. One main concern is the environmental sustainability of energy crop cultivation as large amounts of organic matter and plant nutrients are removed with the crop biomass from the field. Depletion of organic matter and plant nutrients from the agricultural system can lead to soil degradation if not balanced by appropriate replenishments. Secondly, since the number of biogas plants in many European countries has increased significantly in recent years, the disposal of residues from anaerobic digestion has become of growing concern [
10]. From a sustainability perspective of the biogas supply chain, since a wide range of undecomposed organic compounds and plant nutrients removed from the field (mainly ammonia and phosphate) are retained in the digestate [
11,
12,
13,
14], the direct land application of digestate is an economical option for residues disposal and soil amendment/fertilization [
15,
16,
17]. The risk of a potential transfer of organic pollutants, such as herbicides and fungicides, from digestate to rotational crops and feedstuffs is considered very low by the European Food Standards Agency [
16].
A third concern is that energy crops require resources (land, water and energy) which inevitably become no longer available for food production [
8,
9,
18,
19]. For cereal crop-based productions, the “food vs. fuel” conflict would be overcome if the grain was excluded from the biogas feedstock and used for livestock feed, while in general, the conflict would not exist at all if energy-crop cultivation was carried out on soils unsuitable for food production (marginal land) [
20]. In this context, energy crop farming is an effective and profitable strategy to prevent the land from abandonment and degradation while promoting rural investments and new job opportunities [
8].
Digestate can be applied to the soil without further processing (whole digestate, WD) [
17] or after mechanical separation to obtain a solid fibrous material (solid digestate, SD) which can be directly spread to the field, composted, or dried for intermediate storage and transport [
17,
21]. Both WD and SD are sources of organic carbon and plant nutrients but since they exhibit quantitative and qualitative differences, they are expected to contribute differently to soil organic matter turnover [
22], plant nutrients availability, and soil physical properties [
23]. Typically, SD exhibits a great percentage (38–75%) of highly stable organic matter and a low NH
4-N to total-N (TN) ratio [
23], which make it suitable for use as a soil conditioner rather than as a source of readily available N. The use of digestate as a soil amendment can contribute to soil carbon sequestration, especially in intensively cultivated soils where crop residues are removed [
24]. Organic matter addition is beneficial to soil fertility, since it may improve soil structure, increase plant nutrient retention, and water holding capacity and stimulate microbial activity [
25]. A higher microbial activity, in turn, may enhance the release of plant nutrients from added residues and soil organic matter itself [
26]. Conversely, the low organic matter concentration and the high NH
4-N/TN ratio in WD makes it more suitable for use as an N-fertilizer [
22,
23]. The efficiency of digestate as N fertilizer changes with the features of digestate itself, soil type, crop and time of spreading [
4]. Like any other fertilizer, WD should be applied at appropriate rates and times during the crop growing season, to ensure optimum plant nutrient uptake and to avoid phytotoxic effect and pollution of groundwater [
16].
Research on digestate suitability for land application is relatively recent and is focused, on the one hand, on agricultural benefits of digestate as soil fertilizer and/or improver, and on the other hand, on the environmental risks associated with digestate use. Overall, many studies have investigated the potential of digestate as N fertilizer and the fate of N in the soil after land application [
27,
28], as well as the effect of digestate on soil organic matter and chemical properties [
28,
29,
30], while there is still little knowledge about the impact of digestate on soil physical [
27,
28,
31] and biological properties (bacterial and fungal communities) [
32,
33], which are key factors of soil functioning. Knowledge gaps about appropriate rates and soil-digestate interactions still exist, and the research field is very broad and complex, involving different kinds of feedstocks, crops, soils, environments, and agricultural management.
The main goal of our research was to understand the short-term effects of digestate on soil properties through a holistic approach, investigating soil physical, chemical, and microbiological properties and their interactions. Furthermore, we evaluated the effectiveness of digestate in replacing mineral fertilizers and as a resource to compensate for carbon depletion due to biomass removal in a three-year energy crop rotation. The study included both the whole digestate and its mechanically-separated solid fraction.
2. Materials and Methods
2.1. Digestate
The whole digestate was obtained from the biogas plant of the Cooperativa Agroenergetica Territoriale (CAT) in Correggio (Reggio Emilia, Italy). The digester was fed with the above-ground biomass from energy crops, including maize, triticale and sorghum silages, combined with by-products from the agricultural industry (i.e., stalks of grapes and sugar beet pulps), and cattle slurry from Parmesan cheese farms [
34]. The solid fraction (SD) was retrieved from the whole digestate (WD) through a mechanical solid/liquid separation system following the digestion. SD was rich in organic C (44.4% of air-dry digestate) but relatively poor in N, P and K, whereas WD had a very low organic C content (1.1% of air-dry digestate) and a low C/N ratio (3.1), with a large proportion of NH
4-N in the total amount of N (about 60% of air-dry digestate) (
Table S1).
For a more in-depth characterization of digestate, we performed molecular-level analyses of microbial communities (see the paragraph on soil sampling and analysis) on WD, SD and two additional fractions, one collected directly from the fermentation silos, the other one from the mechanically-separated liquor (LD).
2.2. Study Site and Experimental Design
The experimental field was a 35 × 130 m area belonging to the R.G.R. Farm (CAT cooperative partner) located in the lower Po Valley (Correggio, Reggio Emilia, Italy; 44°49′ N–10°45′ E). The land use of the area had been converted from sugar beet cultivation to a 2-year maize-triticale rotation to feed the biogas plant, according to the set-aside scheme introduced by the Common Agriculture Policy. The trial was carried out from January 2011 to October 2013, maize cultivated from spring to summer 2011 and 2013, and triticale from autumn 2011 to summer 2012. The effects of digestate application on soil properties were investigated in the two maize growing seasons, using the whole digestate (WD) as a partial or total replacement of mineral fertilizer, and the digestate solid fraction (SD) as a soil amendment. Nitrogen fertilization was performed during maize post-emergency stage as follows: D0 plots, with mineral fertilizer only (control); D50 plots, based on WD + mineral fertilizer; D100 plots, with WD only. The SD fraction was applied to the WD-fertilized plots (D50 and D100) between one crop cycle and the next.
The treatments were assigned to 4 × 10 m field plots according to a randomized block design with three blocks (replicates). 1 m between plots and 5 m between blocks were kept free to avoid disturbance during soil tillage and to allow machinery operations. During the trial period, the mean annual air temperature was 14.2 °C and precipitation 681 mm (
Figure S1). The experimental soil was a Hypocalcic Hypovertic Calcisols [
35], with a silty-clay texture (
Table S2). The main soil physical and chemical characteristics at the start of the trial (time t
0) are given in
Table S2.
In September 2010, the field was ploughed and harrowed for seedbed preparation. On 2 April 2011, maize (
Zea mais L., cv. Kalumet) was sown at a density of 7 plants/m
2 and all plots fertilized with urea (125 kg N ha
−1). At the plant emergence (20 May 2011), an additional N fertilization was applied as follows: D0 plots, urea (125 kg N ha
−1); D50 plots, urea (62.5 kg N ha
−1) plus WD (17,400 L ha
−1 = 62.5 kg N ha
−1); D100 plots, WD only (34,700 L ha
−1 = 125 kg N ha
−1). WD was spread on the soil surface along the maize rows using mobile equipment (
Figure S2) specifically developed by CAT and Cavazzuti Franco (Carpi, Modena, Italy), consisting of a 1 m
3 tanker mounted on a tractor and connected to a boom with 4 trailing hoses, with a 2.80 m working width. The tanker was equipped with a pump-loading apparatus for filling. Maize was irrigated on 26 May, 13 June and 6 July 2011, and harvested at the wax ripeness stage (17 August 2011). On 16 September 2011, the D50 and D100 plots received 40 m
3 ha
−1 SD (equivalent to 10 t ha
−1), applied by a solid manure spreader (Vaschieri, Solignano di Castelvetro, Modena, Italy) and incorporated into the soil by ploughing and harrowing. Triticale (x
Triticosecale Wittm., cultivar Agrano) was sown in November 2011 at a density of 240 kg seeds ha
−1 and fertilized in a single operation in April 2012 by urea only (30 kg ha
−1). Due to the high plant density and the lack of suitable equipment for WD application in the standing crop, no WD top-dressing treatment was possible for triticale. The option of a pre-sowing WD treatment was discarded because of the low N use efficiency in the autumn-winter period and the related risk of N leaching [
4]. Triticale biomass was harvested on 24 June 2012. In October 2012, the D50 and D100 plots were amended with the SD fraction (40 m
3 ha
−1) and the whole field was prepared for maize sowing as previously described (19 April 2013). The trial continued with a maize cycle according to the same practices as for the first experimental year. Due to unfavourable weather conditions (
Figure S1), sowing, fertilization and harvesting operations needed to be delayed for about one month, respectively. Maize was harvested at the wax ripeness stage on 3 September 2013.
The whole above-ground biomass yielded at the end of the crop cycles was harvested and used as feedstock for biogas production.
The combination of both SD and WD with the agricultural management (fertilization factor) and sampling data (time factor) were the factors considered for the evaluation of differences in soil physical-chemical and biological characteristics.
2.3. Seed Germination Bioassay
Extracts of the two digestate fractions (WD and SD) collected from biogas plant at the beginning of experimentation were prepared by adding 25 g digestate to 100 mL of sterile deionized water. The suspensions were shaken for two hours and then centrifuged at 5000 g for 30 min. The supernatants from each digestate were used to prepare test solutions with digestate concentrations of 100% (pure), 50%, 25%, 12.5% and 0% (distilled water as control). Petri dishes of 9 cm diameter were prepared, each containing twenty maize seeds placed upon two sheets of Whatman N. 1 filter paper pre-treated with 10 mL of the test solution. The dishes were transferred to a germination chamber under controlled temperature (20 °C) in the dark. There were five replicates for each treatment.
The number of seeds germinated in each Petri dish was counted after three days and after one week of incubation, and the germination index (GI) was calculated as a percentage relative to the control [
26]. Seedling root elongation was measured after 1 week.
2.4. Crop Yield
Crop yield just before harvest (in August for maize and in June for triticale) was estimated by collecting biomass at ground level from three randomly selected point in each plot spaced 30 cm from the edges to avoid border effects. In each sampling point, maize was harvested from 1 m in length row sections (including 6–7 plants), while triticale was harvested from 0.5 m2 areas. After weighing, the biomass was oven-dried at 70 °C until constant weight (about 56 h for maize and 48 h for triticale) to determine the dry weight.
2.5. Soil
2.5.1. Sampling
Soil samples were collected before maize sowing (25 March 2011 = t0; at the beginning of the trial); after maize harvesting (17 November 2011 = t1); before sowing in the second maize cycle (14 April 2013 = t2); at the end of the trial (3 October 2013 = t3).
For soil chemical, biochemical, microbiological and particle size analyses, each plot was sampled by auger to a depth of 20 cm in three selected points, collecting soil cores of 5 cm in diameter. The three cores were then mixed thoroughly providing a single composite sample per plot (3 replicates for each treatment, as a whole). Before chemical and biochemical analyses, the soil was air-dried, ground and sieved through a 2 mm mesh size. The samples for microbiological analyses were stored untreated at −80 °C until analysis.
For soil bulk density (BD) and macro-porosity measurements, three undisturbed soil samples were collected from the central part of each plot, at depth increments of 0–10 and 10–20 cm, using a hammer-driven linear sampler. Samples for BD were collected at each sampling time whereas those for macro-porosity analysis were taken only at t0 and t2.
Soil aggregate stability was determined at t0, t1 and t2 on a single composite sample per plot, obtained from three spatially separated sub-samples of soil aggregates collected down to 10 cm depth.
2.5.2. Chemical Analyses
Soil pH was measured potentiometrically in a 1:2.5 soil-water suspension. Soil cation exchange capacity (CEC) and exchangeable base concentrations (Ca, Mg, K and Na) were determined on BaCl
2 triethanolamine (pH 8.2) extracts by flame atomic absorption spectrometry [
36]. Soil available Cu, Zn, Fe and Mn were extracted and quantified according to Lindsay and Norvell [
37]. Soil total organic carbon (TOC) and total nitrogen (TN) in the bulk soil were measured by dry combustion using a Thermo Flash 2000 CN elemental analyser (Thermo Fisher Scientific, Walthman, MA, USA). The analysis was performed on 20 to 40 mg of soil weighed into Ag-foil capsules and pre-treated with 10% HCl until complete removal of carbonates.
2.5.3. Biochemical Analyses
Microbial biomass carbon and nitrogen (MBC and MBN, respectively) were determined following the fumigation extraction method [
38]. Two aliquots from each soil sample were brought to 60% of water holding capacity (WHC), 24 h before the analysis; a first aliquot was immediately extracted with K
2SO
4 (0.5 M) and then filtered with Whatman n. 42 filter paper; the second aliquot was fumigated for 24 h at 25 °C with CHCl
3 and extracted as the first one. The organic C and N concentration in the extracts was then determined by Thermo Flash 2000 CN elemental analyser (Thermo Fisher Scientific). MBC and MBN were calculated as the difference between the C and N extracted from the fumigated samples and those extracted from non-fumigated samples, respectively.
Soil microbial respiration was determined according to Badalucco et al. [
39]. Each sample was incubated at 28 °C in a flask sealed with a stopper. The CO
2 developed during incubation was trapped in NaOH solution after 1, 3, 7, 10, 14, 21 and 28 days and then measured by titration with HCl (0.1 M). The cumulative amount of CO
2 produced over 28 days of incubation (MRcum) was regarded as the potentially mineralizable C.
2.5.4. Microbiological Analyses
Soil RNA was extracted using the RNA PowerSoil
TM Total Isolation Kit (MoBio, Solano Beach, CA, USA), following the manufacturer’s instructions with the minor modification of adding Na-EDTA (0.5 M) to the lyses solution to improve the DNA desorption from clay particles [
40]. RNA was eluted in nuclease-free water (Promega, Madison, WI, USA) and then DNA was co-extracted by the RNA PowerSoil
TM DNA Elution Accessory Kit (MoBio). The extracted RNA was subsequently subjected to DNase digestion using the RQ1 RNase-free DNase (Promega) and complementary cDNA was generated by reverse transcription (RT) using the ImProm-IITM Reverse Transcriptase System (Promega).
For Denaturing Gradient Gel Electrophoresis (DGGE) analysis of microbial communities, the extracted DNA and the generated cDNA were amplified using specific primers for bacterial and archaeal 16S rDNA, and for fungal 18S rDNA (
Table S3). Amplification and DGGE procedures were carried out following Pastorelli et al. [
41] and Lazzaro et al. [
42].
Representative bands from archaea and Clostridiaceae-related DGGEs were excised, eluted from gels and screened according to Pastorelli et al. [
43]. Selected bands were subjected to direct sequencing by Macrogen Service (Macrogen Ltd., Amsterdam, The Netherlands). The nucleotide sequences collected in this study were deposited in the GenBank database under the accession numbers MF415444-MF415489.
2.5.5. Physical Analyses
Soil texture was determined by the pipette method [
44]. Soil bulk density (BD) was measured by the core method according to Blake and Hartge [
45].
Soil macro-porosity was determined by the micro-morphometric method [
46]. This method allows the characterization and quantification of soil macro-porosity according to pore shape, size distribution, irregularity, orientation and continuity from vertically oriented thin sections of 5.5 × 8.5 cm size, obtained from undisturbed soil samples. A 2.82 × 3.54 cm area of each thin section was captured with a video camera avoiding the edges where disruption could have occurred. The images collected were then analysed by Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA), set up specifically to measure pores larger than 50 µm. The total porosity and pore distribution were calculated from the measurement of pore shape and size [
46]. From a functional point of view, the elongated pores of 50–500 µm were described as transmission pores and the pores with >500 µm size as fissures [
47]. The thin sections were also examined by a Zeiss “R POL” microscope at a 25× magnification to characterize soil structure.
Soil aggregate stability was determined by the wet sieving method and the calculation of the mean weight diameter of water-stable aggregates (MWD) [
48]. Soil aggregates from each composite sample were air-dried, weighed and separated into different size fractions (10–20, 4.75–10, 2–4.75, 1–2, <1 mm) using a vibrating sieve shaker (Retsch, Germany). The most representative aggregate size fraction was used to perform wet sieving. Twenty grams of aggregates from the most abundant size class (4.75–10 mm) were directly soaked for 5 min on the top of nests of 4.75, 2, 0.25- and 0.05-mm diameter sieves immersed in water (fast wetting). The nest of sieves with its content was then vertically shaken in water by an electronically controlled machine with a stroke of 40 mm per 10 min, at a rate of 30 complete oscillations per minute. For each sample, 3 repetitions were performed.
2.6. Statistical Analyses
The results of soil physical, chemical and microbiological (richness and α-diversity indices) analyses were processed by analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test at the significance level p ≤ 0.05, using the Statistica software (Palo Alto, CA, USA). Pearson correlation analysis was performed among physico-chemical properties of soil by Statistica software.
Band migration distance and intensity for each DGGE profile were obtained using the Gel Compare II software v 4.6 (Applied Maths, Saint-Martens-Latem, Belgium). The number of bands (species richness) and their relative abundance (Shannon index, H’ and Simpson index, D) were used as proxies of richness and α-diversity of soil microbial communities, as described by Pastorelli et al. [
43]. The banding patterns of bacterial and fungal DGGE profiles were converted into presence/absence band matching tables and imported into PAST3 software [
49]. Non-metric multidimensional scaling (MDS) based on the Dice coefficient was performed to represent the distance between the DGGE profiles in the two-dimensional space. Analysis of similarity (ANOSIM) based on Dice similarity coefficient and 9999 permutational tests were run to assess the statistical significance in microbial community structure due to fertilizer/amendment treatments.
Nucleotide sequence chromatograms were edited using Chromas Lite software v2.1.1 (Technelysium Pty Ltd., Tewantin, Old, AU) to verify the absence of ambiguous peaks and to convert them to FASTA format. The DECIPHER’s Find Chimeras web tool [
50] was used to uncover chimaeras hidden in nucleotide sequences. The Web-based BLAST tools was used to identify closely related nucleotide sequences within those stored in the GenBank database.in order Microbial taxonomic identification was achieved by means of different sequence similarity thresholds as described by Webster et al. [
51].
5. Conclusions
With a focus on the environmental sustainability of the bioenergy supply chain, the application of digestate to the soil can meet the need to safely dispose and recycle the residues coming from anaerobic digestion and, at the same time, to compensate for soil C and plant nutrient depletion due to crop biomass removal.
From our results, digestate application in a three-year maize-triticale rotation cycle proved to be as effective as 100% mineral fertilization in maintaining crop productivity level. Moreover, the increase in soil TOC following digestate treatments confirmed digestate effectiveness to compensate for carbon depletion.
Further research is needed to increase the knowledge about the optimum dose of digestate to be applied in relation to soil/crop specificities and the best application method to minimize potential negative effects of digestate to the soil and environment quality. The pattern and extent with which the effects of digestate treatments were expressed and their temporal fluctuations underline complex dynamics of chemical, physical and biological processes affecting the material brought to the soil. This suggests that a more or less long period of time is needed during which the achievement of a new stable equilibrium in the soil functions is regulated by the interaction between the amount and quality of biomass supplied, the impact of mechanical operations associated with crop management and climate trend.
Further expected benefits from digestate as amendment, such as improvement of soil bulk density and porosity, were not observed, possibly due to a counteracting interference of soil tillage operations. The effectiveness of a soil amendment and the sustainability of the use of digestate can be strongly conditioned by the crop management system as a whole, and in particular, by those cultivation practices that have a direct impact on the soil and the dynamics of the organic matter and nutrients supplied with the treatment. For this reason, to fully exploit digestate potential, its use should be integrated within an overall more conservative soil management system, involving reduced soil mechanical disturbance. This would be essential to prevent soil physical degradation and excessive organic matter mineralization, thus allowing the organic compounds of digestate to perform their chemical, physical and biological functions and minimize the risk of N loss by leaching and/or gas emissions.
Additional considerations regard the cultivation of energy crops in marginal lands or set-aside areas; this could be a solution to the “food vs. fuel conflict” and, at the same time, would promote rural investments and new job opportunities.