A New Method for the Assessment of the Oxidative Potential of Both Water-Soluble and Insoluble PM
Abstract
:1. Introduction
2. Materials and Methods
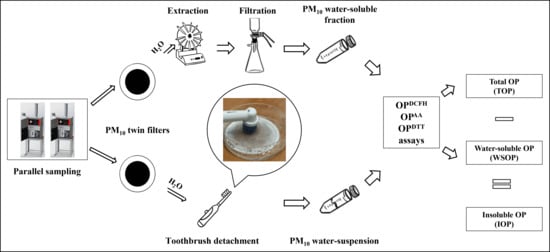
2.1. Sampling Site and Procedure
2.2. Analytical Procedure
2.3. Oxidative Potential Measurements
2.3.1. OPAA
2.3.2. OPDTT
2.3.3. OPDCFH
2.4. Data Analysis
3. Results and Discussion
3.1. WSOP vs. TOP
3.2. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2. 5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide: Executive Summary; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Shaddick, G.; Thomas, M.L.; Mudu, P.; Ruggeri, G.; Gumy, S. Half the world’s population are exposed to increasing air pollution. NPJ Clim. Atmos. Sci. 2020, 3, 23. [Google Scholar] [CrossRef]
- IARC. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Outdoor Air Pollution. IARC Monogr. Eval. Carcinog. Risks Hum. 2016, 109, 9. [Google Scholar]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A., 3rd; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [Green Version]
- Janghorbani, M.; Momeni, F.; Mansourian, M. Systematic review and metaanalysis of air pollution exposure and risk of diabetes. Eur. J. Epidemiol. 2014, 29, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Losacco, C.; Perillo, A. Particulate matter air pollution and respiratory impact on humans and animals. Environ. Sci. Pollut. Res. 2018, 25, 33901–33910. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Cole, T.B.; Dao, K.; Chang, Y.-C.; Coburn, J.; Garrick, J.M. Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol. Ther. 2020, 210, 107523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, H.; Fang, B.; Wang, H.; Yang, Z.; Yang, W.; Hao, Y.; Wang, X.; Wang, Q.; Wang, M. Source Identification and Health Risk Assessment of Polycyclic Aromatic Hydrocarbon-Enriched PM 2.5 in Tangshan, China. Environ. Toxicol. Chem. 2020, 39, 458–467. [Google Scholar] [CrossRef]
- Liu, S.; Jørgensen, J.T.; Ljungman, P.; Pershagen, G.; Bellander, T.; Leander, K.; Magnusson, P.K.; Rizzuto, D.; Hvidtfeldt, U.A.; Raaschou-Nielsen, O.; et al. Long-term exposure to low-level air pollution and incidence of asthma: The ELAPSE project. Eur. Respir. J. 2021, 57, 2003099. [Google Scholar] [CrossRef]
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Q.; Yang, X.; Li, G.; Zhang, J.; Zhou, X.; Jiang, W. Cytotoxicity of the soluble and insoluble fractions of atmospheric fine particulate matter. J. Environ. Sci. 2020, 91, 105–116. [Google Scholar] [CrossRef]
- Øvrevik, J. Oxidative potential versus biological effects: A review on the relevance of cell-free/abiotic assays as predictors of toxicity from airborne particulate matter. Int. J. Mol. Sci. 2019, 20, 4772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Nozza, E.; Valentini, S.; Melzi, G.; Vecchi, R.; Corsini, E. Advances on the immunotoxicity of outdoor particulate matter: A focus on physical and chemical properties and respiratory defence mechanisms. Sci. Total Environ. 2021, 780, 146391. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Mulholland, J.A.; Russell, A.G.; Weber, R.J. Characterization of water-insoluble oxidative potential of PM2.5 using the dithiothreitol assay. Atmos. Environ. 2020, 224, 117327. [Google Scholar] [CrossRef]
- Molina, C.; Toro, A.; Manzano, C.A.R.; Canepari, S.; Massimi, L.; Leiva-Guzmán, M. Airborne aerosols and human health: Leapfrogging from mass concentration to oxidative potential. Atmosphere 2020, 11, 917. [Google Scholar] [CrossRef]
- Bates, J.T.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J.; Tolbert, P.E.; Abrams, J.Y.; Sarnat, S.E.; Klein, M.; Mulholland, J.A.; et al. Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 2019, 53, 4003–4019. [Google Scholar] [CrossRef]
- Borm, P.J.A.; Kelly, F.; Künzli, N.; Schins, R.P.F.; Donaldson, K. Oxidant generation by particulate matter: From biologically effective dose to a promising, novel metric. Occup. Environ. Med. 2007, 64, 73–74. [Google Scholar] [CrossRef] [Green Version]
- Pietrogrande, M.C.; Bacco, D.; Trentini, A.; Russo, M. Effect of filter extraction solvents on the measurement of the oxidative potential of airborne PM2.5. Environ. Sci. Pollut. Res. 2021, 28, 29551–29563. [Google Scholar] [CrossRef]
- Crobeddu, B.; Baudrimont, I.; Deweirdt, J.; Sciare, J.; Badel, A.; Camproux, A.-C.; Bui, L.C.; Baeza-Squiban, A. Lung Antioxidant Depletion: A Predictive Indicator of Cellular Stress Induced by Ambient Fine Particles. Environ. Sci. Technol. 2020, 54, 2360–2369. [Google Scholar] [CrossRef]
- Fang, T.; Verma, V.; Bates, J.T.; Abrams, J.; Klein, M.; Strickland, M.J.; Sarnat, S.E.; Chang, H.H.; Mulholland, J.A.; Tolbert, P.E.; et al. Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: Contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays. Atmos. Chem. Phys. 2016, 16, 3865–3879. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Baumgartner, J.; Zhang, Y.; Wang, Y.; Schauer, J.J. Source apportionment of air pollution exposures of rural Chinese women cooking with biomass fuels. Atmos. Environ. 2015, 104, 79–87. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damagein vivoand in cell culture: How should you do it and what do the results mean? J. Cereb. Blood Flow Metab. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.-H.; Hartner, E.; Utinger, B.; Gfeller, B.; Paul, A.; Sklorz, M.; Czech, H.; Yang, B.X.; Su, X.Y.; Jakobi, G.; et al. Are reactive oxygen species (ROS) a suitable metric to predict toxicity of carbonaceous aerosol particles? Atmos. Chem. Phys. 2021, 22, 1793–1809. [Google Scholar] [CrossRef]
- Daellenbach, K.R.; Uzu, G.; Jiang, J.; Cassagnes, L.-E.; Leni, Z.; Vlachou, A.; Stefenelli, G.; Canonaco, F.; Weber, S.; Segers, A.; et al. Sources of particulate-matter air pollution and its oxidative potential in Europe. Nature 2020, 587, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, G.; Conte, E.; Perrino, C.; Canepari, S. Oxidative potential of size-segregated PM in an urban and an industrial area of Italy. Atmos. Environ. 2018, 187, 292–300. [Google Scholar] [CrossRef]
- Piacentini, D.; Falasca, G.; Canepari, S.; Massimi, L. Potential of PM-selected components to induce oxidative stress and root system alteration in a plant model organism. Environ. Int. 2019, 132, 105094. [Google Scholar] [CrossRef]
- Conte, E.; Canepari, S.; Frasca, D.; Simonetti, G. Oxidative potential of selected PM components. Multidiscip. Digit. Publ. Inst. Proc. 2017, 1, 108. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.; Zhang, L.; Wang, X.; Xie, T.; Zhou, S.; Lu, S.; Liu, X.; Lu, H.; Xiao, K.; Wang, W.; et al. Oxidative Potential Induced by Ambient Particulate Matters with Acellular assays: A Review. Processes 2020, 8, 1410. [Google Scholar] [CrossRef]
- Gao, D.; Fang, T.; Verma, V.; Zeng, L.; Weber, R.J. A method for measuring total aerosol oxidative potential (OP) with the dithiothreitol (DTT) assay and comparisons between an urban and roadside site of water-soluble and total OP. Atmos. Meas. Tech. 2017, 10, 2821–2835. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Fang, T.; Xu, L.; Peltier, R.E.; Russell, A.G.; Ng, N.L.; Weber, R.J. Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5. Environ. Sci. Technol. 2015, 49, 4646–4656. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, F.; Qu, F.; Ding, W. Water-insoluble fraction of airborne particulate matter (PM10) induces oxidative stress in human lung epithelial A549 cells. Environ. Toxicol. 2012, 29, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Zeng, L.; Gao, D.; Verma, V.; Stefaniak, A.B.; Weber, R.J. Ambient Size Distributions and Lung Deposition of Aerosol Dithiothreitol-Measured Oxidative Potential: Contrast between Soluble and Insoluble Particles. Environ. Sci. Technol. 2017, 51, 6802–6811. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ripley, S.; Weichenthal, S.; Pollitt, K.J.G. Ambient particulate matter oxidative potential: Chemical determinants, associated health effects, and strategies for risk management. Free Radic. Biol. Med. 2020, 151, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, A.; Limbeck, A. Recent developments in assessment of bio-accessible trace metal fractions in airborne particulate matter: A review. Anal. Chim. Acta 2013, 774, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, J.; Li, J.; Jiang, N.; Zhang, R.; Yang, W.; Yao, W.; Wu, W. Oxidative stress and endocytosis are involved in upregulation of interleukin-8 expression in airway cells exposed to PM2.5. Environ. Toxicol. 2016, 31, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Shi, T.; Borm, P.J.; Schins, R.P. Soluble metals as well as the insoluble particle fraction are involved in cellular DNA damage induced by particulate matter. In Oxygen/Nitrogen Radicals: Cell Injury and Disease; Springer: Boston, MA, USA, 2002; pp. 317–326. [Google Scholar]
- Soukup, J.M.; Becker, S. Human Alveolar Macrophage Responses to Air Pollution Particulates Are Associated with Insoluble Components of Coarse Material, Including Particulate Endotoxin. Toxicol. Appl. Pharmacol. 2001, 171, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Daher, N.; Ning, Z.; Cho, A.K.; Shafer, M.; Schauer, J.J.; Sioutas, C. Comparison of the Chemical and Oxidative Characteristics of Particulate Matter (PM) Collected by Different Methods: Filters, Impactors, and BioSamplers. Aerosol Sci. Technol. 2011, 45, 1294–1304. [Google Scholar] [CrossRef]
- McWhinney, R.D.; Badali, K.; Liggio, J.; Li, S.-M.; Abbatt, J.P.D. Filterable Redox Cycling Activity: A Comparison between Diesel Exhaust Particles and Secondary Organic Aerosol Constituents. Environ. Sci. Technol. 2013, 47, 3362–3369. [Google Scholar] [CrossRef]
- Frezzini, M.A.; De Francesco, N.; Massimi, L.; Canepari, S. Effects of operating conditions on PM oxidative potential assays. Atmos. Environ. 2022, 268, 118802. [Google Scholar] [CrossRef]
- Wang, D.; Pakbin, P.; Shafer, M.M.; Antkiewicz, D.; Schauer, J.J.; Sioutas, C. Macrophage reactive oxygen species activity of water-soluble and water-insoluble fractions of ambient coarse, PM2.5 and ultrafine particulate matter (PM) in Los Angeles. Atmos. Environ. 2013, 77, 301–310. [Google Scholar] [CrossRef]
- Fuller, S.; Wragg, F.; Nutter, J.; Kalberer, M. Comparison of on-line and off-line methods to quantify reactive oxygen species (ROS) in atmospheric aerosols. Atmos. Environ. 2014, 92, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Rico-Martinez, R.; Kotra, N.; King, L.; Liu, J.; Snell, T.W.; Weber, R.J. Contribution of Water-Soluble and Insoluble Components and Their Hydrophobic/Hydrophilic Subfractions to the Reactive Oxygen Species-Generating Potential of Fine Ambient Aerosols. Environ. Sci. Technol. 2012, 46, 11384–11392. [Google Scholar] [CrossRef] [PubMed]
- Massimi, L.; Astolfi, M.L.; Canepari, S. Simple and Efficient Method to Detach Intact PM10 from Field Filters: Elements Recovery Assessment. 2022; Under Review. [Google Scholar]
- Süring, K.; Bach, S.; Höflich, C.; Straff, W. Flow Cytometric Analysis of Particle-bound Bet v 1 Allergen in PM10. J. Vis. Exp. 2016, 117, e54721. [Google Scholar] [CrossRef] [Green Version]
- Süring, K.; Bach, S.; Bossmann, K.; Wolter, E.; Neumann, A.; Straff, W.; Höflich, C. PM10 contains particle-bound allergens: Dust analysis by Flow Cytometry. Environ. Technol. Innov. 2016, 5, 60–66. [Google Scholar] [CrossRef]
- Massimi, L.; Simonetti, G.; Buiarelli, F.; Di Filippo, P.; Pomata, D.; Riccardi, C.; Ristorini, M.; Astolfi, M.L.; Canepari, S. Spatial distribution of levoglucosan and alternative biomass burning tracers in atmospheric aerosols, in an urban and industrial hotspot of Central Italy. Atmos. Res. 2020, 239, 104904. [Google Scholar] [CrossRef]
- Massimi, L.; Ristorini, M.; Simonetti, G.; Frezzini, M.A.; Astolfi, M.L.; Canepari, S. Spatial mapping and size distribution of oxidative potential of particulate matter released by spatially disaggregated sources. Environ. Pollut. 2020, 266, 115271. [Google Scholar] [CrossRef] [PubMed]
- ASRO. SR EN 12341: 2014. Air Quality–Determination of the PM10 Fraction of Suspended Particulate Matter. Reference Method and Field Test Procedure to Demonstrate Reference Equivalence of Measurements Methods. 2014. Available online: https://magazin.asro.ro/en/standard/8734 (accessed on 25 January 2022).
- Canepari, S.; Cardarelli, E.; Pietrodangelo, A.; Strincone, M. Determination of metals, metalloids and non-volatile ions in airborne particulate matter by a new two-step sequential leaching procedure Part A: Experimental design and optimization. Taanta 2006, 69, 581–587. [Google Scholar] [CrossRef]
- Canepari, S.; Cardarelli, E.; Pietrodangelo, A.; Strincone, M. Determination of metals, metalloids and non-volatile ions in air-borne particulate matter by a new two-step sequential leaching procedure: Part B: Validation on equivalent real samples. Talanta 2006, 69, 588–595. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Protano, C.; Marconi, E.; Massimi, L.; Brunori, M.; Piamonti, D.; Migliara, G.; Vitali, M.; Canepari, S. A new rapid treatment of human hair for elemental determination by inductively coupled mass spectrometry. Anal. Methods 2020, 12, 1906–1918. [Google Scholar] [CrossRef]
- Conti, M.E.; Iacobucci, M.; Cucina, D.; Mecozzi, M. Multivariate statistical methods applied to biomonitoring studies. Int. J. Environ. Pollut. 2007, 29, 333. [Google Scholar] [CrossRef]
- Massimi, L.; Ristorini, M.; Eusebio, M.; Florendo, D.; Adeyemo, A.; Brugnoli, D.; Canepari, S. Monitoring and Evaluation of Terni (Central Italy) Air Quality through Spatially Resolved Analyses. Atmosphere 2017, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Calas, A.; Uzu, G.; Kelly, F.J.; Houdier, S.; Martins, J.M.F.; Thomas, F.; Molton, F.; Charron, A.; Dunster, C.; Oliete, A.; et al. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France). Atmos. Chem. Phys. 2018, 18, 7863–7875. [Google Scholar] [CrossRef] [Green Version]
- Janssen, N.A.; Yang, A.; Strak, M.; Steenhof, M.; Hellack, B.; Gerlofs-Nijland, M.E.; Kuhlbusch, T.; Kelly, F.; Harrison, R.; Brunekreef, B.; et al. Oxidative potential of particulate matter collected at sites with different source characteristics. Sci. Total Environ. 2014, 472, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Massimi, L.; Pietrodangelo, A.; Frezzini, M.A.; Ristorini, M.; De Francesco, N.; Sargolini, T.; Amoroso, A.; Di Giosa, A.; Canepari, S.; Perrino, C. Effects of COVID-19 lockdown on PM10 composition and sources in the Rome Area (Italy) by elements’ chemical fractionation-based source apportionment. Atmos. Res. 2021, 266, 105970. [Google Scholar] [CrossRef]
- Lokorai, K.; Ali-Khodja, H.; Khardi, S.; Bencharif-Madani, F.; Naidja, L.; Bouziane, M. Influence of mineral dust on the concentration and composition of PM10 in the city of Constantine. Aeolian Res. 2021, 50, 100677. [Google Scholar] [CrossRef]
- Canepari, S.; Astolfi, M.; Catrambone, M.; Frasca, D.; Marcoccia, M.; Marcovecchio, F.; Massimi, L.; Rantica, E.; Perrino, C. A combined chemical/size fractionation approach to study winter/summer variations, ageing and source strength of atmospheric particles. Environ. Pollut. 2019, 253, 19–28. [Google Scholar] [CrossRef]
- Perrone, M.R.; Bertoli, I.; Romano, S.; Russo, M.; Rispoli, G.; Pietrogrande, M.C. PM2.5 and PM10 oxidative potential at a Central Mediterranean Site: Contrasts between dithiothreitol- and ascorbic acid-measured values in relation with particle size and chemical composition. Atmos. Environ. 2019, 210, 143–155. [Google Scholar] [CrossRef]
- Owoade, K.O.; Hopke, P.; Olise, F.S.; Ogundele, L.T.; Fawole, O.; Olaniyi, B.H.; Jegede, O.O.; Ayoola, M.A.; Bashiru, M.I. Chemical compositions and source identification of particulate matter (PM 2.5 and PM 2.5–10) from a scrap iron and steel smelting industry along the Ife–Ibadan highway, Nigeria. Atmos. Pollut. Res. 2015, 6, 107–119. [Google Scholar] [CrossRef]
- Harrison, R.M.; Allan, J.; Carruthers, D.; Heal, M.R.; Lewis, A.C.; Marner, B.; Murrells, T.; Williams, A. Non-exhaust vehicle emissions of particulate matter and VOC from road traffic: A review. Atmos. Environ. 2021, 262, 118592. [Google Scholar] [CrossRef]
- Hicks, W.; Beevers, S.; Tremper, A.H.; Stewart, G.; Priestman, M.; Kelly, F.J.; Lanoiselle, M.; Lowry, D.; Green, D.C. Quanti-fication of non-exhaust particulate matter traffic emissions and the impact of COVID-19 lockdown at London Marylebone Road. Atmosphere 2021, 12, 190. [Google Scholar] [CrossRef]
- Massimi, L.; Ristorini, M.; Astolfi, M.L.; Perrino, C.; Canepari, S. High resolution spatial mapping of element concentrations in PM10: A powerful tool for localization of emission sources. Atmos. Res. 2020, 244, 105060. [Google Scholar] [CrossRef]
- Charrier, J.G.; Anastasio, C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: Evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012, 12, 9321–9333. [Google Scholar] [CrossRef] [Green Version]
- King, L.E.; Weber, R.J. Development and testing of an online method to measure ambient fine particulate reactive oxygen species (ROS) based on the 2’,7’-dichlorofluorescin (DCFH) assay. Atmos. Meas. Tech. 2013, 6, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Charron, A.; Polo-Rehn, L.; Besombes, J.-L.; Golly, B.; Buisson, C.; Chanut, H.; Marchand, N.; Guillaud, G.; Jaffrezo, J.-L. Identification and quantification of particulate tracers of exhaust and non-exhaust vehicle emissions. Atmos. Chem. Phys. 2019, 19, 5187–5207. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, A.; Harrison, R.M. Sources and properties of non-exhaust particulate matter from road traffic: A review. Sci. Total Environ. 2008, 400, 270–282. [Google Scholar] [CrossRef]
- Li, W.; Ge, P.; Chen, M.; Tang, J.; Cao, M.; Cui, Y.; Hu, K.; Nie, D. Tracers from Biomass Burning Emissions and Identification of Biomass Burning. Atmosphere 2021, 12, 1401. [Google Scholar] [CrossRef]
- Pietrogrande, M.C.; Bertoli, I.; Clauser, G.; Dalpiaz, C.; Dell’Anna, R.; Lazzeri, P.; Lenzi, W.; Russo, M. Chemical composition and oxidative potential of atmospheric particles heavily impacted by residential wood burning in the alpine region of northern Italy. Atmos. Environ. 2021, 253, 118360. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [Green Version]
- Pant, P.; Harrison, R.M. Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmos. Environ. 2013, 77, 78–97. [Google Scholar] [CrossRef]
- Canepari, S.; Perrino, C.; Olivieri, F.; Astolfi, M.L. Characterisation of the traffic sources of PM through size-segregated sampling, sequential leaching and ICP analysis. Atmos. Environ. 2008, 42, 8161–8175. [Google Scholar] [CrossRef]
- Guo, H.-B.; Li, M.; Lyu, Y.; Cheng, T.-T.; Xv, J.; Li, X. Size-resolved particle oxidative potential in the office, laboratory, and home: Evidence for the importance of water-soluble transition metals. Environ. Pollut. 2019, 246, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, L.; Salvatori, E.; Winkler, A.; Frezzini, M.A.; De Santis, E.; Sagnotti, L.; Canepari, S.; Manes, F. Urban trees for bio-monitoring atmospheric particulate matter: An integrated approach combining plant functional traits, magnetic and chemical properties. Ecol. Indic. 2021, 126, 107707. [Google Scholar] [CrossRef]
- Calas, A.; Uzu, G.; Besombes, J.-L.; Martins, J.M.; Redaelli, M.; Weber, S.; Charron, A.; Albinet, A.; Chevrier, F.; Brulfert, G.; et al. Seasonal Variations and Chemical Predictors of Oxidative Potential (OP) of Particulate Matter (PM), for Seven Urban French Sites. Atmosphere 2019, 10, 698. [Google Scholar] [CrossRef] [Green Version]
- Pietrogrande, M.C.; Russo, M.; Zagatti, E. Review of PM Oxidative Potential Measured with Acellular Assays in Urban and Rural Sites across Italy. Atmosphere 2019, 10, 626. [Google Scholar] [CrossRef] [Green Version]
- Hakimzadeh, M.; Soleimanian, E.; Mousavi, A.; Borgini, A.; De Marco, C.; Ruprecht, A.A.; Sioutas, C. The impact of biomass burning on the oxidative potential of PM2.5 in the metropolitan area of Milan. Atmos. Environ. 2020, 224, 117328. [Google Scholar] [CrossRef]
- Hedayat, F.; Stevanovic, S.; Miljevic, B.; Bottle, S.; Ristovski, Z. Review-evaluating the molecular assays for measuring the oxidative potential of particulate matter. Chem. Ind. Chem. Eng. Q. 2015, 21, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Altuwayjiri, A.; Pirhadi, M.; Kalafy, M.; Alharbi, B.; Sioutas, C. Impact of different sources on the oxidative potential of ambient particulate matter PM10 in Riyadh, Saudi Arabia: A focus on dust emissions. Sci. Total Environ. 2022, 806, 150590. [Google Scholar] [CrossRef]
- Almeida, S.M.; Pio, C.A.; Freitas, M.C.; Reis, M.A.; Trancoso, M.A. Source apportionment of fine and coarse particulate matter in a sub-urban area at the Western European Coast. Atmos. Environ. 2005, 39, 3127–3138. [Google Scholar] [CrossRef]



| Water-Soluble Fraction (ws) | Insoluble Fraction (i) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PM10 | Al | Cr | Cs | Cu | Fe | Li | Ni | Rb | Sb | Sn | Al | Cr | Cs | Cu | Fe | Li | Ni | Rb | Sb | Sn | |
| UoM | µg·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 | ng·m−3 |
| MDL | 5 | 1.7 | 0.063 | 0.00076 | 0.033 | 3.3 | 0.045 | 0.031 | 0.018 | 0.051 | 0.46 | 4.7 | 1.9 | 0.00054 | 0.44 | 22 | 0.018 | 0.71 | 0.039 | 0.074 | 0.11 |
| 05/05/2021 | 26 | 21 | 2.5 | 0.017 | 0.73 | 7.6 | 0.27 | 0.4 | 0.34 | 0.059 | 0.065 | 178 | 97 | 0.058 | 10 | 661 | 0.22 | 31 | 0.39 | 0.39 | 1.1 |
| 06/05/2021 | 37 | 23 | 1.6 | 0.027 | 0.71 | 6.9 | 0.90 | 0.38 | 0.45 | 0.048 | 0.048 | 447 | 135 | 0.15 | 20 | 1157 | 1.3 | 59 | 0.97 | 0.55 | 2 |
| 07/05/2021 | 36 | 25 | 4 | 0.025 | 1.7 | 9 | 0.45 | 0.8 | 0.43 | 0.14 | 0.076 | 371 | 246 | 0.14 | 23 | 1618 | 0.041 | 74 | 0.95 | 1 | 2.5 |
| 08/05/2021 | 20 | 29 | 1.8 | 0.011 | 1.3 | 10 | 0.22 | 0.53 | 0.2 | 0.14 | 0.36 | 117 | 52 | 0.035 | 7 | 441 | 0.12 | 23 | 0.23 | 0.32 | 1.5 |
| 09/05/2021 | 24 | 30 | 2.6 | 0.01 | 1.1 | 12 | 0.12 | 0.69 | 0.36 | 0.14 | 0.16 | 143 | 69 | 0.039 | 13 | 402 | 0.13 | 37 | 0.45 | 0.24 | 0.99 |
| 10/05/2021 | 21 | 10 | 1.1 | 0.012 | 0.93 | 10 | 0.15 | 0.41 | 0.41 | 0.2 | 0.13 | 148 | 52 | 0.051 | 6.7 | 306 | 0.15 | 9 | 0.59 | 0.3 | 0.68 |
| 11/05/2021 | 21 | 63 | 4.1 | 0.028 | 1.5 | 16 | 0.68 | 0.6 | 0.7 | 0.23 | 0.22 | 355 | 109 | 0.11 | 10 | 879 | 0.4 | 24 | 0.71 | 0.68 | 1.5 |
| 12/05/2021 | 25 | 20 | 2.2 | 0.029 | 1.4 | 8.2 | 0.46 | 0.47 | 0.41 | 0.051 | 0.041 | 224 | 114 | 0.072 | 17 | 944 | 0.3 | 52 | 0.43 | 0.5 | 1.7 |
| 13/05/2021 | 29 | 11 | 2.2 | 0.022 | 1.3 | 7.1 | 0.66 | 0.68 | 0.37 | 0.05 | 0.05 | 158 | 44 | 0.034 | 9 | 384 | 0.34 | 21 | 0.26 | 0.22 | 0.68 |
| 14/05/2021 | 20 | 28 | 2.3 | 0.029 | 1.3 | 8.6 | 0.17 | 1.1 | 0.38 | 0.11 | 0.24 | 124 | 202 | 0.033 | 23 | 1101 | 0.11 | 184 | 0.16 | 0.46 | 2.2 |
| 15/05/2021 | 18 | 7.2 | 1.2 | 0.009 | 0.42 | 5.5 | 0.05 | 0.33 | 0.16 | 0.10 | 0.043 | 51 | 39 | 0.011 | 4.1 | 227 | 0.041 | 15 | 0.11 | 0.19 | 0.52 |
| 16/05/2021 | 13 | 25 | 2 | 0.012 | 0.79 | 9 | 0.15 | 0.45 | 0.33 | 0.084 | 0.04 | 277 | 508 | 0.058 | 31 | 2791 | 0.23 | 151 | 0.45 | 0.88 | 2.8 |
| 17/05/2021 | N.D. | 28 | 1.5 | 0.035 | 0.19 | 8.3 | 0.57 | 0.15 | 0.55 | 0.043 | 0.031 | 472 | 153 | 0.13 | 14 | 945 | 0.8 | 40 | 0.71 | 0.51 | 2.2 |
| 18/05/2021 | N.D. | 24 | 2.7 | 0.019 | 0.88 | 7.8 | 0.28 | 0.31 | 0.25 | 0.062 | 0.072 | 121 | 63 | 0.038 | 6.8 | 409 | 0.22 | 13 | 0.23 | 0.25 | 0.8 |
| 19/05/2021 | 20 | 15 | 1.1 | 0.0067 | 0.35 | 3.4 | 0.17 | 0.13 | 0.14 | 0.048 | 0.15 | 57 | 16 | 0.013 | 1.8 | 138 | 0.078 | 3.9 | 0.15 | 0.11 | 0.34 |
| 20/05/2021 | 10 | 58 | 1.2 | 0.015 | 0.72 | 8.6 | 0.40 | 0.35 | 0.3 | 0.052 | 0.044 | 333 | 101 | 0.069 | 10 | 604 | 0.56 | 34 | 0.54 | 0.36 | 0.92 |
| 21/05/2021 | 33 | 56 | 3.2 | 0.038 | 1.8 | 15 | 1.15 | 0.92 | 0.6 | 0.15 | 0.11 | 228 | 102 | 0.081 | 16 | 796 | 0.36 | 64 | 0.46 | 0.42 | 1.3 |
| 22/05/2021 | 31 | 14 | 2 | 0.012 | 0.91 | 12 | 0.11 | 0.35 | 0.31 | 0.45 | 0.083 | 182 | 88 | 0.046 | 5.5 | 344 | 0.17 | 10 | 0.39 | 0.51 | 0.71 |
| 23/05/2021 | 17 | 26 | 5.2 | 0.01 | 1.1 | 13 | 0.30 | 0.49 | 0.37 | 0.24 | 0.084 | 377 | 181 | 0.084 | 12 | 1185 | 0.39 | 36 | 0.62 | 0.65 | 1.3 |
| 24/05/2021 | N.D. | 9 | 2.1 | 0.012 | 0.9 | 8.3 | 0.15 | 0.51 | 0.24 | 0.1 | 0.074 | 127 | 95 | 0.034 | 5.3 | 335 | 0.13 | 7.1 | 0.34 | 0.24 | 0.56 |
| Mean | 24 | 26 | 2.3 | 0.019 | 1 | 9 | 0.37 | 0.5 | 0.36 | 0.12 | 0.11 | 225 | 123 | 0.064 | 12 | 783 | 0.3 | 44 | 0.46 | 0.44 | 1.3 |
| Std. Dev. | 7.7 | 16 | 1.1 | 0.01 | 0.43 | 3.1 | 0.29 | 0.24 | 0.14 | 0.1 | 0.085 | 127 | 107 | 0.04 | 7.5 | 613 | 0.29 | 47 | 0.25 | 0.24 | 0.72 |
| DCFH | AA | DTT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WSOP | TOP | IOP | IOP/TOP | WSOP | TOP | IOP | IOP/TOP | WSOP | TOP | IOP | IOP/TOP | |
| UoM | nmol H2O2 m−3 | % | nmol AA min−1·m−3 | % | nmol DTT min−1·m−3 | % | ||||||
| MDL | 1.1 × 10−10 | 0.0096 | 0.0058 | |||||||||
| 05/05/2021 | 1.1 × 10−8 | 2.2 × 10−8 | 1.1 × 10−8 | 49 | 0.37 | 0.42 | 0.051 | 12 | 0.32 | 0.49 | 0.17 | 35 |
| 06/05/2021 | 6.8 × 10−9 | 1.9 × 10−8 | 1.2 × 10−8 | 63 | 0.25 | 1.2 | 0.99 | 80 | 0.077 | 0.79 | 0.71 | 90 |
| 07/05/2021 | 1.1 × 10−8 | 2.2 × 10−8 | 1.1 × 10−8 | 51 | 1.6 | 2.1 | 0.36 | 18 | 0.43 | 0.82 | 0.39 | 48 |
| 08/05/2021 | 1.8 × 10−8 | 1.9 × 10−8 | 4.7 × 10−10 | 2 | 0.16 | 0.94 | 0.78 | 83 | 0.63 | 0.97 | 0.34 | 35 |
| 09/05/2021 | 1.7 × 10−8 | 2.1 × 10−8 | 3.9 × 10−9 | 18 | 0.67 | 1.4 | 0.69 | 51 | 0.75 | 0.89 | 0.14 | 15 |
| 10/05/2021 | 1.1 × 10−8 | 1.2 × 10−8 | 8.7 × 10−10 | 8 | 0.69 | 1.9 | 1.2 | 64 | 0.86 | 0.86 | 0.0015 | 0.17 |
| 11/05/2021 | 3.4 × 10−8 | 4.1 × 10−8 | 6.6 × 10−9 | 16 | 0.28 | 1.6 | 1.3 | 82 | 0.61 | 1.4 | 0.83 | 58 |
| 12/05/2021 | 8.2 × 10−9 | 2.1 × 10−8 | 1.2 × 10−8 | 59 | 1.5 | 1.7 | 0.25 | 15 | 0.19 | 0.22 | 0.02 | 9 |
| 13/05/2021 | 6.9 × 10−9 | 1.2 × 10−8 | 5.2 × 10−9 | 43 | 0.69 | 1.5 | 0.84 | 55 | 0.52 | 0.71 | 0.19 | 27 |
| 14/05/2021 | 1.9 × 10−8 | 1.9 × 10−8 | 4.4 × 10−10 | 2 | 0.46 | 1.5 | 1.1 | 69 | 0.46 | 0.84 | 0.38 | 45 |
| 15/05/2021 | 1.5 × 10−9 | 2.4 × 10−8 | 2.3 × 10−8 | 94 | 0.62 | 1.5 | 0.89 | 59 | 0.51 | 1.1 | 0.49 | 49 |
| 16/05/2021 | 9.3 × 10−9 | 9.9 × 10−9 | 6.3 × 10−10 | 6 | 0.04 | 0.89 | 0.86 | 96 | 0.26 | 0.59 | 0.33 | 56 |
| 17/05/2021 | 7.4 × 10−9 | 1.9 × 10−8 | 1.2 × 10−8 | 62 | 0.47 | 0.91 | 0.44 | 48 | 0.11 | 1.1 | 0.96 | 90 |
| 18/05/2021 | 1.5 × 10−8 | 3.1 × 10−8 | 1.5 × 10−8 | 50 | 1.1 | 1.2 | 0.18 | 15 | 0.39 | 0.78 | 0.39 | 50 |
| 19/05/2021 | 1.4 × 10−8 | 1.8 × 10−8 | 4.3 × 10−9 | 23 | 0.33 | 0.92 | 0.59 | 64 | 0.0011 | 0.72 | 0.72 | 99 |
| 20/05/2021 | 1.9 × 10−8 | 1.9 × 10−8 | 1.1 × 10−9 | 5 | 0.14 | 0.58 | 0.44 | 76 | 0.079 | 0.11 | 0.021 | 21 |
| 21/05/2021 | 2.2 × 10−8 | 3.3 × 10−8 | 1.6 × 10−8 | 42 | 0.96 | 1.6 | 0.65 | 41 | 1.19 | 1.2 | 0.022 | 1.8 |
| 22/05/2021 | 1.9 × 10−8 | 2.9 × 10−8 | 9.8 × 10−9 | 33 | 0.42 | 0.62 | 0.21 | 33 | 0.056 | 0.81 | 0.75 | 93 |
| 23/05/2021 | 1.8 × 10−8 | 1.8 × 10−8 | 8.1 × 10−10 | 4 | 0.59 | 3.9 | 3.3 | 85 | 0.41 | 0.51 | 0.11 | 22 |
| 24/05/2021 | 1.5 × 10−8 | 2.4 × 10−8 | 8.9 × 10−9 | 37 | 0.59 | 1.9 | 1.3 | 69 | 0.04 | 0.31 | 0.27 | 86 |
| Mean | 1.4 × 10−8 | 2.2 × 10−8 | 7.6 × 10−9 | 35 | 0.59 | 1.4 | 0.82 | 58 | 0.39 | 0.76 | 0.36 | 48 |
| Std. Dev. | 7.1 × 10−9 | 7.7 × 10−9 | 6.2 × 10−9 | 81 | 0.42 | 0.74 | 0.69 | 93 | 0.31 | 0.32 | 0.3 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frezzini, M.A.; Di Iulio, G.; Tiraboschi, C.; Canepari, S.; Massimi, L. A New Method for the Assessment of the Oxidative Potential of Both Water-Soluble and Insoluble PM. Atmosphere 2022, 13, 349. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos13020349
Frezzini MA, Di Iulio G, Tiraboschi C, Canepari S, Massimi L. A New Method for the Assessment of the Oxidative Potential of Both Water-Soluble and Insoluble PM. Atmosphere. 2022; 13(2):349. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos13020349
Chicago/Turabian StyleFrezzini, Maria Agostina, Gianluca Di Iulio, Caterina Tiraboschi, Silvia Canepari, and Lorenzo Massimi. 2022. "A New Method for the Assessment of the Oxidative Potential of Both Water-Soluble and Insoluble PM" Atmosphere 13, no. 2: 349. https://0-doi-org.brum.beds.ac.uk/10.3390/atmos13020349