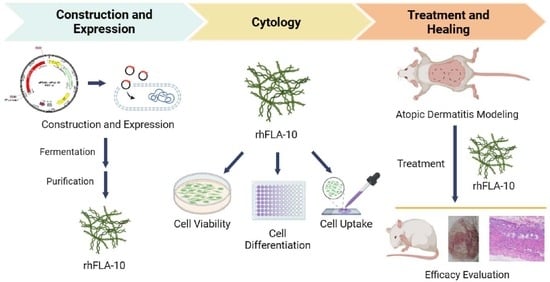
A Novel Recombinant Human Filaggrin Segment (rhFLA-10) Alleviated a Skin Lesion of Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Recombinant Human Filaggrin (rhFLA-10) Expression Vector
2.2. Expression and Identification of rhFLA-10
2.3. Purification and Purity Identification of rhFLA-10
2.4. Effect of rhFLA-10 on HaCaT Cell Viability
2.5. β-Hexosaminidase Assay
2.6. Neutral Red Staining Assay
2.7. Expression of M1-Type Macrophage Markers
2.8. Uptake of rhFLA-10 by HaCaT
2.9. Percutaneous Penetration of rhFLA-10 in C57BL/6 Mice
2.10. Induction of Atopic Dermatitis Model and Treatment with rhFLA-10 Gel
2.11. Mouse Scratching Behavior Assay
2.12. Detection of the Extent of Skin Lesions in Mice
2.13. Histopathologic Examination of the Skin
2.14. Statistical Analysis
3. Results
3.1. Expression and Purification of rhFLA-10
3.2. rhFLA-10 Inhibited the Degranulation of Mast Cells
3.3. Effect of rhFLA-10 on Phenotypic Differentiation of m1-Type Macrophages
3.4. Uptake Behavior of rhFLA-10 in HaCaT Cells
3.5. Transdermal Absorption Properties of rhFLA-10 in Mice
3.6. rhFLA-10 Treatment Study of Atopic Dermatitis in KM Mice
3.7. Histopathologic Section Study of Skin Lesions in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weidinger, S.; Beck, L.; Bieber, T.; Kabashima, K.; Irvine, A. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K. The global burden of skin disease in 2010: An analysis of the prevalence and impact of skin conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Stefanovic, N.; Irvine, A.D. Filaggrin and beyond: New insights into the skin barrier in atopic dermatitis and allergic diseases, from genetics to therapeutic perspectives. Ann. Allergy Asthma Immunol. 2023, 132, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Cho, S.-Y.; Kwon, J.; Hwang, M.; Hwang, H.; Kang, Y.J.; Lee, H.-S.; Kim, J.; Kim, W.K. Anti-atopic dermatitis effects of Parasenecio auriculatus via simultaneous inhibition of multiple inflammatory pathways. BMB Rep. 2022, 55, 275. [Google Scholar] [CrossRef]
- Zein, N.M. “Cpdx”, a Non-Steroidal Selective Glucocorticoid Receptor Agonistic Modulator (SEGRAM) Selectively Triggers the Beneficial Anti-Inflammatory Activity of Glucocorticoids, but Not Their Long-Term Debilitating Effects. Ph.D. Thesis, Université de Strasbourg, Strasbourg, France, 2018. [Google Scholar]
- Lu, H.-F.; Zhou, Y.-C.; Cheng, B.-H. Involvement and repair of epithelial barrier dysfunction in allergic diseases. Front. Immunol. 2024, 15, 1348272. [Google Scholar] [CrossRef]
- Gruber, R.; Elias, P.M.; Crumrine, D.; Lin, T.-K.; Brandner, J.M.; Hachem, J.-P.; Presland, R.B.; Fleckman, P.; Janecke, A.R.; Sandilands, A. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am. J. Pathol. 2011, 178, 2252–2263. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Cork, M.J.; Amagai, M.; De Benedetto, A.; Kabashima, K.; Hamilton, J.D.; Rossi, A.B. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov. 2022, 2, 100131. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Berger, T.; Fassett, M. Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 239–250. [Google Scholar] [CrossRef]
- Liu, A.W.; Gillis, J.E.; Sumpter, T.L.; Kaplan, D.H. Neuroimmune interactions in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1169–1177. [Google Scholar] [CrossRef]
- Elias, P.M.; Gruber, R.; Crumrine, D.; Menon, G.; Williams, M.L.; Wakefield, J.S.; Holleran, W.M.; Uchida, Y. Formation and functions of the corneocyte lipid envelope (CLE). Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 314–318. [Google Scholar] [CrossRef]
- Kezic, S.; Jakasa, I. Filaggrin and skin barrier function. Ski. Barrier Funct. 2016, 49, 1–7. [Google Scholar]
- Kezic, S.; O’regan, G.; Yau, N.; Sandilands, A.; Chen, H.; Campbell, L.; Kroboth, K.; Watson, R.; Rowland, M.; McLean, W.I. Levels of filaggrin degradation products are influenced by both filaggrin genotype and atopic dermatitis severity. Allergy 2011, 66, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Basu, M.; Mortz, C.; Jensen, T.; Barington, T.; Halken, S. Natural moisturizing factors in children with and without eczema: Associations with lifestyle and genetic factors. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Armengot-Carbo, M.; Hernández-Martín, Á.; Torrelo, A. The role of filaggrin in the skin barrier and disease development. Actas Dermo-Sifiliográficas Engl. Ed. 2015, 106, 86–95. [Google Scholar] [CrossRef] [PubMed]
- David Boothe, W.; Tarbox, J.A.; Tarbox, M.B. Atopic dermatitis: Pathophysiology. In Management of Atopic Dermatitis: Methods and Challenges; Springer: Cham, Switzerland, 2017; Volume 1027, pp. 21–37. [Google Scholar]
- McAleer, M.A.; Irvine, A.D. The multifunctional role of filaggrin in allergic skin disease. J. Allergy Clin. Immunol. 2013, 131, 280–291. [Google Scholar] [CrossRef]
- Irvine, A.D.; McLean, W.I. Breaking the (un) sound barrier: Filaggrin is a major gene for atopic dermatitis. J. Investig. Dermatol. 2006, 126, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, M.F.; Kanchan, K.; Bahnson, H.T.; Ruczinski, I.; Shankar, G.; Leung, D.Y.; Baloh, C.; Toit, G.D.; Lack, G.; Nepom, G.T. Epidermal differentiation complex genetic variation in atopic dermatitis and peanut allergy. J. Allergy Clin. Immunol. 2023, 151, 1137–1142.e4. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Mizawa, M.; Takemoto, K.; Yamamoto, S.; Shimizu, T. Altered expression of s100 fused-type proteins in an atopic dermatitis skin model. Exp. Dermatol. 2023, 32, 2160–2165. [Google Scholar] [CrossRef] [PubMed]
- Budu-Aggrey, A.; Kilanowski, A.; Sobczyk, M.K.; Team, R.; Shringarpure, S.S.; Mitchell, R.; Reis, K.; Reigo, A.; Team, E.B.R.; Mägi, R. European and multi-ancestry genome-wide association meta-analysis of atopic dermatitis highlights importance of systemic immune regulation. Nat. Commun. 2023, 14, 6172. [Google Scholar] [CrossRef]
- Mildner, M.; Jin, J.; Eckhart, L.; Kezic, S.; Gruber, F.; Barresi, C.; Stremnitzer, C.; Buchberger, M.; Mlitz, V.; Ballaun, C. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J. Investig. Dermatol. 2010, 130, 2286–2294. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Huang, S.; Yu, F.; Bei, Y.; Zhang, Y.; Tang, J.; Huang, Y.; Xiang, Q. Hybrid freeze-dried dressings composed of epidermal growth factor and recombinant human-like collagen enhance cutaneous wound healing in rats. Front. Bioeng. Biotechnol. 2020, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Wu, Q.; Kuang, Z.; Cong, J.; Zhang, Q.; Huang, Y.; Su, Z.; Xiang, Q. Temperature-controlled expression of a recombinant human-like collagen I peptide in Escherichia coli. Bioengineering 2023, 10, 926. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Geng, D.; Zhang, Q.; Ye, T.; Zhang, Y.; Li, Z.; Huang, Y.; Xiang, Q. Recombinant expression a novel fibronectin—Collage fusion peptide modulating stem cell stemness via integrin β3. Appl. Microbiol. Biotechnol. 2022, 106, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, W.; Lei, X.; Luo, X.; Xiang, Q.; Zhu, X.; Pan, Q.; Jin, P.; Cheng, B. Treatment of acute wounds with recombinant human-like collagen and recombinant human-like fibronectin in c57bl/6 mice individually or in combination. Front. Bioeng. Biotechnol. 2022, 10, 908585. [Google Scholar] [CrossRef]
- Bryksin, A.V.; Matsumura, I. Overlap extension PCR cloning: A simple and reliable way to create recombinant plasmids. Biotechniques 2010, 48, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.; Masone, B.S.; Ribbe, J. One-step purification of recombinant proteins with the 6xhis tag and NI-NTA resin. Basic DNA RNA Protoc. 1996, 58, 491–510. [Google Scholar]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage m1/m2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.A.; Byrne, H.J. Modification of the in vitro uptake mechanism and antioxidant levels in HaCat cells and resultant changes to toxicity and oxidative stress of G4 and G6 poly (amidoamine) dendrimer nanoparticles. Anal. Bioanal. Chem. 2016, 408, 5295–5307. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, M.; Boisgard, A.-S.; Danoy, A.; El Kholti, N.; Salvi, J.-P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Advanced characterization of imiquimod-induced psoriasis-like mouse model. Pharmaceutics 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed]
- Andoh, T.; Haza, S.; Saito, A.; Kuraishi, Y. Involvement of leukotriene b4 in spontaneous itch-related behaviour in nc mice with atopic dermatitis-like skin lesions. Exp. Dermatol. 2011, 20, 894–898. [Google Scholar] [CrossRef]
- Matsuda, H. Development of atopic adrmatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 491. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Spriestersbach, A.; Kubicek, J.; Schäfer, F.; Block, H.; Maertens, B. Purification of his-tagged proteins. Methods Enzymol. 2015, 559, 1–15. [Google Scholar] [PubMed]
- Singha, T.K.; Gulati, P.; Mohanty, A.; Khasa, Y.P.; Kapoor, R.K.; Kumar, S. Efficient genetic approaches for improvement of plasmid based expression of recombinant protein in Escherichia coli: A review. Process Biochem. 2017, 55, 17–31. [Google Scholar] [CrossRef]
- Sahid, M.N.A.; Kiyoi, T. Mast cell activation markers for in vitro study. J. Immunoass. Immunochem. 2020, 41, 778–816. [Google Scholar] [CrossRef]
- Frazier, W.; Bhardwaj, N. Atopic dermatitis: Diagnosis and treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar]
- Kim, Y.; Lim, K.-M. Skin barrier dysfunction and filaggrin. Arch. Pharmacal Res. 2021, 44, 36–48. [Google Scholar] [CrossRef]
- Ahmad, I.; Nawaz, N.; Darwesh, N.M.; Rahman, S.U.; Mustafa, M.Z.; Khan, S.B.; Patching, S.G. Overcoming challenges for amplified expression of recombinant proteins using Escherichia coli. Protein Expr. Purif. 2018, 144, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, D.S.; Mandlik, S.K. Atopic dermatitis: New insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol. Immunotoxicol. 2021, 43, 105–125. [Google Scholar] [CrossRef]
- Hou, D.-D.; Gu, Y.-J.; Wang, D.-C.; Niu, Y.; Xu, Z.-R.; Jin, Z.-Q.; Wang, X.-X.; Li, S.-J. Therapeutic effects of myricetin on atopic dermatitis in vivo and in vitro. Phytomedicine 2022, 102, 154200. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Li, W.; Hu, Q.; Huang, H.; Xu, X.; Du, B.; Li, P. Probiotic-fermented Portulaca oleracea L. Alleviated DNFB-induced atopic dermatitis by inhibiting the NF-κB signaling pathway. J. Ethnopharmacol. 2023, 313, 116613. [Google Scholar] [CrossRef] [PubMed]





| Observation Item | Score | Score Standard |
|---|---|---|
| Skin thickness | 0 | Smooth skin without folds |
| 1 | The skin appears slightly wrinkled | |
| 2 | The skin is all slightly wrinkled | |
| 3 | The skin folds further | |
| 4 | Severe wrinkling accompanied by weight loss or poor condition | |
| Chippings covering condition | 0 | The skin is smooth without scales |
| 1 | The skin appears slightly scaly | |
| 2 | Slightly scaly skin covered | |
| 3 | The skin is completely covered with scales and thickened | |
| 4 | Heavy chippings accompanied by weight loss or poor condition | |
| Erythema dermis | 0 | The skin is smooth |
| 1 | The skin has slight erythema | |
| 2 | The skin is completely covered with erythema | |
| 3 | The severity of erythema is further deepened | |
| 4 | Severe erythema with weight loss or poor condition |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Zhong, X.; Liao, H.; Cong, J.; Wu, Q.; Liang, S.; Xiang, Q. A Novel Recombinant Human Filaggrin Segment (rhFLA-10) Alleviated a Skin Lesion of Atopic Dermatitis. Bioengineering 2024, 11, 426. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering11050426
Zhu J, Zhong X, Liao H, Cong J, Wu Q, Liang S, Xiang Q. A Novel Recombinant Human Filaggrin Segment (rhFLA-10) Alleviated a Skin Lesion of Atopic Dermatitis. Bioengineering. 2024; 11(5):426. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering11050426
Chicago/Turabian StyleZhu, Jiawen, Xinhua Zhong, Hui Liao, Jianhang Cong, Qiqi Wu, Shuang Liang, and Qi Xiang. 2024. "A Novel Recombinant Human Filaggrin Segment (rhFLA-10) Alleviated a Skin Lesion of Atopic Dermatitis" Bioengineering 11, no. 5: 426. https://0-doi-org.brum.beds.ac.uk/10.3390/bioengineering11050426