Characterization of Morphologically Distinct Components in the Tarsal Secretion of Medauroidea extradentata (Phasmatodea) Using Cryo-Scanning Electron Microscopy
Abstract
:1. Introduction
- (I)
- It can increase the adhesion to a broad range of substrates. Experiments on the attachment performance of Phasmatodea [48] and Coleoptera [49], where the volume of the tarsal secretion was diminished through consecutive steps or by porous substrates, showed that the attachment forces were enhanced on smooth surfaces, but were reduced on rough surfaces, indicating that the fluid is a crucial part of attachment generation on rough surfaces [48,49]. This effect was additionally supported by experiments on the bioinspired micropatterned samples [50]. These results indicate that the secretion can fill the asperities of non-smooth substrates, thus increasing the real contact area and thereby the attachment forces [32,48,51,52,53]. The immersion of nanometric beads in the accumulated tarsal secretions of the beetle Coccinella septempunctata and the fly Calliphora vicina indicated different viscosities of 21.8 and 10.9 mPa × s, respectively, showing that the physical properties of the fluid diverge between the species [33]. The presence of the liquid in contact is expected to provide capillary forces that increase adhesion [4,7]. Additionally, the high viscosity of the fluid likely implements viscous forces and thereby increases attachment [54].
- (II)
- It contributes to decontamination. Contaminating the adhesive pads of the stick insect Carausius morosus with polystyrene beads and manipulating the amount of adhesive fluid showed that a high amount of fluid led to a faster recovery rate of adhesion than a low fluid amount. Thus, it is an important part of the self-cleaning mechanism of smooth adhesive pads [30,31].
- (III)
- It can compensate for different surface chemistry of substrates. Chemical analyses of the fluids allow for the interpretation of the interaction with different surfaces. Due to the presence of two phases (water-soluble and lipid-soluble phases), the emulsion should improve the attachment to hydrophilic and hydrophobic surfaces, as it acts as a coupling agent between the pad and substrates with different free surface energies [7,28,52,55].
2. Materials and Methods
2.1. Animals
2.2. Footprint Collection
2.3. Cryo-Scanning Electron Microscopy
2.4. White Light Interferometry (WLI)
2.5. Temperature and Ambient Humidity Measurements
3. Results
3.1. Analysis of Frozen Footprints
3.2. Distribution of the Tarsal Secretion and Solid Bodies within the Footprints
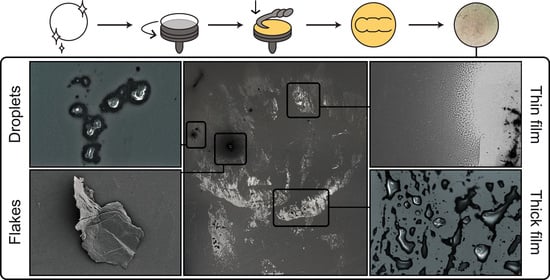
3.3. Droplets
3.4. Flakes
3.5. Thin Films
3.6. Thick Films
3.7. Contaminations
3.8. Evaporation Rates
3.9. Light Microscopy Observations
4. Discussion
4.1. Possible Origin of the Flake Component
4.2. Self-Cleaning Mechanism
4.3. Attachment
4.4. Viscosity and Capillary Forces
4.5. Free Surface Energy
4.6. Leveling Substrate Asperities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creton, C.; Gorb, S. Sticky Feet: From Animals to Materials. MRS Bull. 2007, 32, 466–472. [Google Scholar] [CrossRef]
- Eisner, T.; Aneshansley, D.J. Defense by foot adhesion in a beetle (Hemisphaerota cyanea). Proc. Natl. Acad. Sci. USA 2000, 97, 6568–6573. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Schuppert, J.M.; Dattinger, S.; Gorb, S.N. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J. Insect Physiol. 2008, 54, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Ditsche, P.; Summers, A.P. Aquatic versus terrestrial attachment: Water makes a difference. Beilstein J. Nanotechnol. 2014, 5, 2424–2439. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.H.; Gorb, S.N. Physical constraints lead to parallel evolution of micro- and nanostructures of animal adhesive pads: A review. Beilstein J. Nanotechnol. 2021, 12, 725–743. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Convergent Evolution of Animal Adhesive Pads. In Convergent Evolution; Springer: Cham, Switzerland, 2023; pp. 257–287. [Google Scholar] [CrossRef]
- Gorb, S.N. Smooth Attachment Devices in Insects: Functional Morphology and Biomechanics. Adv. Insect Physiol. 2007, 34, 81–115. [Google Scholar] [CrossRef]
- Gorb, S.N.; Beutel, R.G. Evolution of locomotory attachment pads of hexapods. Naturwissenschaften 2001, 88, 530–534. [Google Scholar] [CrossRef]
- Federle, W. Why are so many adhesive pads hairy? J. Exp. Biol. 2006, 209, 2611–2621. [Google Scholar] [CrossRef]
- Autumn, K.; Sitti, M.; Liang, Y.A.; Peattie, A.M.; Hansen, W.R.; Sponberg, S.; Kenny, T.W.; Fearing, R.; Israelachvili, J.N.; Full, R.J. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci. USA 2002, 99, 12252–12256. [Google Scholar] [CrossRef]
- Dirks, J.-H.; Federle, W. Fluid-based adhesion in insects—Principles and challenges. Soft Matter 2011, 7, 11047–11053. [Google Scholar] [CrossRef]
- Dirks, J.-H.; Federle, W. Mechanisms of fluid production in smooth adhesive pads of insects. J. R. Soc. Interface 2011, 8, 952–960. [Google Scholar] [CrossRef]
- Busshardt, P.; Wolf, H.; Gorb, S.N. Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 2012, 115, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M. Biological Adhesives; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Davey, P.A.; Power, A.M.; Santos, R.; Bertemes, P.; Ladurner, P.; Palmowski, P.; Clarke, J.; Flammang, P.; Lengerer, B.; Hennebert, E.; et al. Omics-based molecular analyses of adhesion by aquatic invertebrates. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1051–1075. [Google Scholar] [CrossRef]
- Delroisse, J.; Kang, V.; Gouveneaux, A.; Santos, R.; Flammang, P. Convergent Evolution of Attachment Mechanisms in Aquatic Animals. In Convergent Evolution; Springer: Cham, Switzerland, 2023; pp. 523–557. [Google Scholar] [CrossRef]
- Federle, W.; Barnes, W.J.P.; Baumgartner, W.; Drechsler, P.; Smith, J.M. Wet but not slippery: Boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 2006, 3, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Langowski, J.K.A.; Dodou, D.; Kamperman, M.; van Leeuwen, J.L. Tree frog attachment: Mechanisms, challenges, and perspectives. Front. Zool. 2018, 15, 32. [Google Scholar] [CrossRef]
- Rasmussen, M.H.; Holler, K.R.; Baio, J.E.; Jaye, C.; Fischer, D.A.; Gorb, S.N.; Weidner, T. Evidence that gecko setae are coated with an ordered nanometre-thin lipid film. Biol. Lett. 2022, 18, 20220093. [Google Scholar] [CrossRef] [PubMed]
- Biederman-Thorson, M.A. Biological Mechanisms of Attachment: The Comparative Morphology and Bioengineering of Organs for Linkage, Suction, and Adhesion; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar]
- Flammang, P.; Michel, A.; Cauwenberge, A.V.; Alexandre, H.; Jangoux, M. A study of the temporary adhesion of the podia in the sea star asterias rubens (Echinodermata, asteroidea) through their footprints. J. Exp. Biol. 1998, 201 Pt 16, 2383–2395. [Google Scholar] [CrossRef]
- Flammang, P. Adhesive Secretions in Echinoderms: An Overview. In Biological Adhesives: With 15 Tables; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 183–206. [Google Scholar]
- Hennebert, E.; Maldonado, B.; Ladurner, P.; Flammang, P.; Santos, R. Experimental strategies for the identification and characterization of adhesive proteins in animals: A review. Interface Focus 2015, 5, 20140064. [Google Scholar] [CrossRef]
- Seabra, S.; Zenleser, T.; Grosbusch, A.L.; Hobmayer, B.; Lengerer, B. The Involvement of Cell-Type-Specific Glycans in Hydra Temporary Adhesion Revealed by a Lectin Screen. Biomimetics 2022, 7, 166. [Google Scholar] [CrossRef]
- Betz, O. Adhesive Exocrine Glands in Insects: Morphology, Ultrastructure, and Adhesive Secretion. In Biological Adhesive Systems; Springer: Vienna, Austria, 2010; pp. 111–152. [Google Scholar] [CrossRef]
- Schmitt, C.; Betz, O. Morphology and ultrastructure of the tarsal adhesive organs of the Madagascar hissing cockroach Gromphadorhina portentosa. Cell Tissue Res. 2017, 370, 243–265. [Google Scholar] [CrossRef]
- Gorb, S.S.N. Attachment Devices of Insect Cuticle; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Vötsch, W.; Nicholson, G.; Müller, R.; Stierhof, Y.-D.; Gorb, S.; Schwarz, U. Chemical composition of the attachment pad secretion of the locust Locusta migratoria. Insect Biochem. Mol. Biol. 2002, 32, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Abou, B.; Gay, C.; Laurent, B.; Cardoso, O.; Voigt, D.; Peisker, H.; Gorb, S. Extensive collection of femtolitre pad secretion droplets in the beetle Leptinotarsa decemlineata allows nanolitre microrheology. J. R. Soc. Interface 2010, 7, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.J.; Bullock, J.M.R.; Beale, A.; Federle, W. Evidence for self-cleaning in fluid-based smooth and hairy adhesive systems of insects. J. Exp. Biol. 2010, 213, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Clemente, C.J.; Federle, W. Mechanisms of self-cleaning in fluid-based smooth adhesive pads of insects. Bioinspir. Biomim. 2012, 7, 046001. [Google Scholar] [CrossRef]
- Dirks, J.-H. Adhesion in Insects. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–10. [Google Scholar]
- Peisker, H.; Heepe, L.; Kovalev, A.E.; Gorb, S.N. Comparative study of the fluid viscosity in tarsal hairy attachment systems of flies and beetles. J. R. Soc. Interface 2014, 11, 20140752. [Google Scholar] [CrossRef]
- Betz, O.; Albert, K.; Boley, M.; Frenzel, M.; Gerhardt, H.; Grunwald, I.; Hartwig, A.; Kleemeier, M.; Maurer, A.; Neuenfeldt, M.; et al. Struktur und Funktion des tarsalen Haftsystems der Madagaskar-Fauchschabe Grompadorhina portentosa (Blattodea). Mitt. Dtsch. Ges. Allg. Angew. Ent. 2018, 21, 159–164. [Google Scholar]
- Kaimaki, D.-M.; Andrew, C.N.S.; Attipoe, A.E.L.; Labonte, D. The physical properties of the stick insect pad secretion are independent of body size. J. R. Soc. Interface 2022, 19, 20220212. [Google Scholar] [CrossRef]
- Bauchhen, E. Die Pulvillen von Calliphora erythrocephala (Diptera, Brachycera) als Adhsionsorgane. Zoomorphology 1979, 93, 99–123. [Google Scholar] [CrossRef]
- Schmitt, U. Hydrocarbons in tarsal glands of Bombus terrestris. Experientia 1990, 46, 1080–1082. [Google Scholar] [CrossRef]
- Eltz, T. Tracing pollinator footprints on natural flowers. J. Chem. Ecol. 2006, 32, 907–915. [Google Scholar] [CrossRef]
- Ishii, S. Adhesion of a Leaf Feeding Ladybird Epilachna vigintioctomaculta (Coleoptera: Coccinellidae) on a Vertically Smooth Surface. Appl. Entomol. Zool. 1987, 22, 222–228. [Google Scholar] [CrossRef]
- Kosaki, A.; Yamaoka, R. Chemical Composition of Footprints and Cuticula Lipids of Three Species of Lady Beetles. Jpn. J. Appl. Entomol. Zool. 1996, 40, 47–53. [Google Scholar] [CrossRef]
- Betz, O. Structure of the tarsi in some Stenus species (Coleoptera, Staphylinidae): External morphology, ultrastructure, and tarsal secretion. J. Morphol. 2003, 255, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Geiselhardt, S.F.; Geiselhardt, S.; Peschke, K. Comparison of tarsal and cuticular chemistry in the leaf beetle Gastrophysa viridula (Coleoptera: Chrysomelidae) and an evaluation of solid-phase microextraction and solvent extraction techniques. Chemoecology 2009, 19, 185–193. [Google Scholar] [CrossRef]
- Attygale, A.B.; Aneshansley, D.J.; Meinwald, J.; Eisner, T. Defense by foot adhesion in a chrysomelid beetle (Hemisphaerota cyanea): Characterization of the adhesive oil. Zoology 2000, 103, 1–6. [Google Scholar]
- Geiselhardt, S.F.; Federle, W.; Prüm, B.; Geiselhardt, S.; Lamm, S.; Peschke, K. Impact of chemical manipulation of tarsal liquids on attachment in the Colorado potato beetle, Leptinotarsa decemlineata. J. Insect Physiol. 2010, 56, 398–404. [Google Scholar] [CrossRef]
- Geiselhardt, S.F.; Lamm, S.; Gack, C.; Peschke, K. Interaction of liquid epicuticular hydrocarbons and tarsal adhesive secretion in Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). J. Comp. Physiol. A 2010, 196, 369–378. [Google Scholar] [CrossRef]
- Geiselhardt, S.F.; Geiselhardt, S.; Peschke, K. Congruence of epicuticular hydrocarbons and tarsal secretions as a principle in beetles. Chemoecology 2011, 21, 181–186. [Google Scholar] [CrossRef]
- Reitz, M.; Gerhardt, H.; Schmitt, C.; Betz, O.; Albert, K.; Lämmerhofer, M. Analysis of chemical profiles of insect adhesion secretions by gas chromatography–mass spectrometry. Anal. Chim. Acta 2015, 854, 47–60. [Google Scholar] [CrossRef]
- Drechsler, P.; Federle, W. Biomechanics of smooth adhesive pads in insects: Influence of tarsal secretion on attachment performance. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2006, 192, 1213–1222. [Google Scholar] [CrossRef]
- Gorb, E.V.; Hosoda, N.; Miksch, C.; Gorb, S.N. Slippery pores: Anti-adhesive effect of nanoporous substrates on the beetle attachment system. J. R. Soc. Interface 2010, 7, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, A.E.; Varenberg, M.; Gorb, S.N. Wet versus dry adhesion of biomimetic mushroom-shaped microstructures. Soft Matter 2012, 8, 7560–7566. [Google Scholar] [CrossRef]
- Barnes, W.J.; Smith, J.; Oines, C.; Mundl, R. Bionics and wet grip. Tire Technol. Int. 2002, 2002, 56–60. [Google Scholar]
- Dirks, J.-H.; Clemente, C.J.; Federle, W. Insect tricks: Two-phasic foot pad secretion prevents slipping. J. R. Soc. Interface 2010, 7, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Bullock, J.M.R.; Drechsler, P.; Federle, W. Comparison of smooth and hairy attachment pads in insects: Friction, adhesion and mechanisms for direction-dependence. J. Exp. Biol. 2008, 211, 3333–3343. [Google Scholar] [CrossRef]
- Stefan, J. Versuche über die scheinbare Adhäsion. Ann. Phys. Chem. 1875, 230, 316–318. [Google Scholar] [CrossRef]
- Thomas, J.; Gorb, S.N.; Büscher, T.H. Influence of surface free energy of the substrate and flooded water on the attachment performance of stick insects (Phasmatodea) with different adhesive surface microstructures. J. Exp. Biol. 2023, 226, jeb244295. [Google Scholar] [CrossRef]
- Lockey, K.H. Lipids of the insect cuticle: Origin, composition and function. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 89, 595–645. [Google Scholar] [CrossRef]
- Gorb, S.; Jiao, Y.; Scherge, M. Ultrastructural architecture and mechanical properties of attachment pads in Tettigonia viridissima (Orthoptera Tettigoniidae). J. Comp. Physiol. A 2000, 186, 821–831. [Google Scholar] [CrossRef]
- Gorb, S.; Scherge, M. Biological microtribology: Anisotropy in frictional forces of orthopteran attachment pads reflects the ultrastructure of a highly deformable material. Proc. Biol. Sci. 2000, 267, 1239–1244. [Google Scholar] [CrossRef]
- Phasmida Species File Online. Available online: http://phasmida.speciesfile.org/HomePage/Phasmida/HomePage.aspx (accessed on 11 August 2023).
- Büscher, T.H.; Buckley, T.R.; Grohmann, C.; Gorb, S.N.; Bradler, S. The Evolution of Tarsal Adhesive Microstructures in Stick and Leaf Insects (Phasmatodea). Front. Ecol. Evol. 2018, 6, 69. [Google Scholar] [CrossRef]
- Büscher, T.H.; Kryuchkov, M.; Katanaev, V.L.; Gorb, S.N. Versatility of Turing patterns potentiates rapid evolution in tarsal attachment microstructures of stick and leaf insects (Phasmatodea). J. R. Soc. Interface 2018, 15, 20180281. [Google Scholar] [CrossRef]
- Büscher, T.H.; Gorb, S.N. Complementary effect of attachment devices in stick insects (Phasmatodea). J. Exp. Biol. 2019, 222, jeb209833. [Google Scholar] [CrossRef]
- Büscher, T.H.; Grohmann, C.; Bradler, S.; Gorb, S.N. Tarsal Attachment Pads in Phasmatodia (Hexapoda: Insecta); Schweizerbart Science Publishers: Stuttgart, Germany, 2019. [Google Scholar]
- Labonte, D.; Federle, W. Functionally different pads on the same foot allow control of attachment: Stick insects have load-sensitive “heel” pads for friction and shear-sensitive “toe” pads for adhesion. PLoS ONE 2013, 8, e81943. [Google Scholar] [CrossRef] [PubMed]
- Büscher, T.H.; Becker, M.; Gorb, S.N. Attachment performance of stick insects (Phasmatodea) on convex substrates. J. Exp. Biol. 2020, 223, jeb226514. [Google Scholar] [CrossRef]
- Labonte, D.; Williams, J.A.; Federle, W. Surface contact and design of fibrillar ‘friction pads’ in stick insects (Carausius morosus): Mechanisms for large friction coefficients and negligible adhesion. J. R. Soc. Interface 2014, 11, 20140034. [Google Scholar] [CrossRef]
- Winand, J.; Gorb, S.N.; Büscher, T.H. Gripping performance in the stick insect Sungaya inexpectata in dependence on the pretarsal architecture. J. Comp. Physiol. A 2023, 209, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Burack, J.; Gorb, S.N.; Büscher, T.H. Attachment Performance of Stick Insects (Phasmatodea) on Plant Leaves with Different Surface Characteristics. Insects 2022, 13, 952. [Google Scholar] [CrossRef]
- Peattie, A.M.; Dirks, J.-H.; Henriques, S.; Federle, W. Arachnids secrete a fluid over their adhesive pads. PLoS ONE 2011, 6, e20485. [Google Scholar] [CrossRef]
- Fowler, J.E.; Gorb, S.; Baio, J.E. Multi-Technique Investigation of a Biomimetic Insect Tarsal Adhesive Fluid. Front. Mech. Eng. 2021, 7, 681120. [Google Scholar] [CrossRef]
- Dirks, J.-H. Physical principles of fluid-mediated insect attachment—Shouldn’t insects slip? Beilstein J. Nanotechnol. 2014, 5, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Federle, W.; Riehle, M.; Curtis, A.S.G.; Full, R.J. An integrative study of insect adhesion: Mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 2002, 42, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Peisker, H.; Gorb, S.N. Evaporation dynamics of tarsal liquid footprints in flies (Calliphora vicina)and beetles (Coccinella septempunctata). J. Exp. Biol. 2012, 215, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, W.; Li, S. Random loose packing of small particles with liquid cohesion. AIChE J. 2019, 65, 500–511. [Google Scholar] [CrossRef]
- Kendall, K. Molecular Adhesion and Its Applications: The Sticky Universe; Kluwer Academic Publishers: Boston, MA, USA, 2004. [Google Scholar]
- Pelofsky, A.H. Surface Tension-Viscosity Relation for Liquids. J. Chem. Eng. Data 1966, 11, 394–397. [Google Scholar] [CrossRef]
- Zheng, M.; Tian, J.; Mulero, Á. New correlations between viscosity and surface tension for saturated normal fluids. Fluid Phase Equilibria 2013, 360, 298–304. [Google Scholar] [CrossRef]
- Gorb, E.; Gorb, S. Effects of surface topography and chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol. Exp. Appl. 2009, 130, 222–228. [Google Scholar] [CrossRef]
- Gorb, E.V.; Gorb, S.N. Anti-adhesive effects of plant wax coverage on insect attachment. J. Exp. Bot. 2017, 68, 5323–5337. [Google Scholar] [CrossRef]
- Labonte, D.; Robinson, A.; Bauer, U.; Federle, W. Disentangling the role of surface topography and intrinsic wettability in the prey capture mechanism of Nepenthes pitcher plants. Acta Biomater. 2021, 119, 225–233. [Google Scholar] [CrossRef]
- Bullock, J.M.R.; Federle, W. The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci. 2011, 18, 298–304. [Google Scholar] [CrossRef]
- England, M.W.; Sato, T.; Yagihashi, M.; Hozumi, A.; Gorb, S.N.; Gorb, E.V. Surface roughness rather than surface chemistry essentially affects insect adhesion. Beilstein J. Nanotechnol. 2016, 7, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Gorb, S.N. Biological Fibrillar Adhesives: Functional Principles and Biomimetic Applications. In Handbook of Adhesion Technology; Da Silva, L.F.M., Öchsner, A., Adams, R.D., Eds.; Springer: Berlin/Heidelberg, Germany; Cham, Switzerland, 2011; pp. 1409–1436. [Google Scholar]
- Büscher, T.H.; Quigley, E.; Gorb, S.N. Adhesion Performance in the Eggs of the Philippine Leaf Insect Phyllium philippinicum (Phasmatodea: Phylliidae). Insects 2020, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Salerno, G.; Rebora, M.; Piersanti, S.; Büscher, T.H.; Gorb, E.V.; Gorb, S.N. Oviposition site selection and attachment ability of Propylea quatuordecimpunctata and Harmonia axyridis from the egg to the adult stage. Physiol. Entomol. 2022, 47, 20–37. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, J.; Gorb, S.N.; Büscher, T.H. Characterization of Morphologically Distinct Components in the Tarsal Secretion of Medauroidea extradentata (Phasmatodea) Using Cryo-Scanning Electron Microscopy. Biomimetics 2023, 8, 439. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics8050439
Thomas J, Gorb SN, Büscher TH. Characterization of Morphologically Distinct Components in the Tarsal Secretion of Medauroidea extradentata (Phasmatodea) Using Cryo-Scanning Electron Microscopy. Biomimetics. 2023; 8(5):439. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics8050439
Chicago/Turabian StyleThomas, Julian, Stanislav N. Gorb, and Thies H. Büscher. 2023. "Characterization of Morphologically Distinct Components in the Tarsal Secretion of Medauroidea extradentata (Phasmatodea) Using Cryo-Scanning Electron Microscopy" Biomimetics 8, no. 5: 439. https://0-doi-org.brum.beds.ac.uk/10.3390/biomimetics8050439