The Impact of Extracellular Ca2+ and Nanosecond Electric Pulses on Sensitive and Drug-Resistant Human Breast and Colon Cancer Cells
Abstract
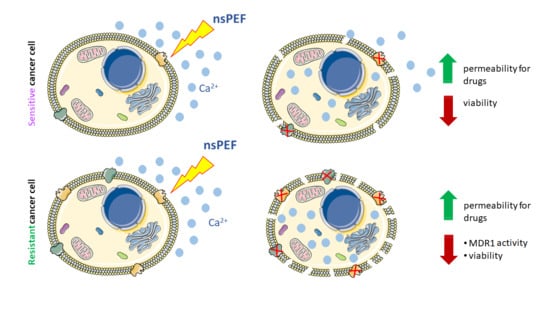
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pulsed Power Setup and PEFs Protocols
2.2. Cell Culture
2.3. The Evaluation of Calcium and Magnesium Ion Content in Experimental Buffers
2.4. Cell Permeabilization Rate Evaluation Using Flow Cytometry
2.5. The Effect of PEF and Extracellular Ca2+
2.6. Viability Assay
2.7. P-glycoprotein and Cell Membrane Fluorescence Imaging
2.8. Rhodamine 123 Accumulation Studies—A Marker of P-gp Activity
2.9. Statistical Analysis
3. Results
3.1. Cells’ Susceptibility to Membrane Electropermeabilization
3.2. PEF Effects with/without Calcium Ions on Cell Viability
3.3. The Effect of PEF Combined with Calcium Ions on P-glycoprotein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omran, Z.; Scaife, P.; Stewart, S.; Rauch, C. Physical and biological characteristics of multi drug resistance (MDR): An integral approach considering pH and drug resistance in cancer. Semin. Cancer Biol. 2017, 43, 42–48. [Google Scholar] [CrossRef]
- Ye, S.; MacEachran, D.P.; Hamilton, J.W.; O’Toole, G.A.; Stanton, B.A. Chemotoxicity of doxorubicin and surface expression of P-glycoprotein (MDR1) is regulated by the Pseudomonas aeruginosa toxin Cif. Am. J. Physiol. Physiol. 2008, 295, C807–C818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, F.; Chu, S.; Bence, A.K.; Bailey, B.; Xue, X.; Erickson, P.A.; Montrose, M.H.; Beck, W.T.; Erickson, L.C. Quantitation of Doxorubicin Uptake, Efflux, and Modulation of Multidrug Resistance (MDR) in MDR Human Cancer Cells. J. Pharmacol. Exp. Ther. 2008, 324, 95 LP–102 LP. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [Green Version]
- Briffa, R.; Langdon, S.P.; Grech, G.; Harrison, D.J. Acquired and Intrinsic Resistance to Colorectal Cancer Treatment. In Colorectal Cancer—Diagnosis, Screening and Management; InTechOpen: London, UK, 2017. [Google Scholar]
- Dartier, J.; Lemaitre, E.; Chourpa, I.; Goupille, C.; Servais, S.; Chevalier, S.; Mahéo, K.; Dumas, J.F. ATP-dependent activity and mitochondrial localization of drug efflux pumps in doxorubicin-resistant breast cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1075–1084. [Google Scholar] [CrossRef]
- Pan, S.T.; Li, Z.L.; He, Z.X.; Qiu, J.X.; Zhou, S.F. Molecular mechanisms for tumour resistance to chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC transporters as mediators of drug resistance and contributors to cancer cell biology. Drug Resist. Updat. 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Hu, T.; Li, Z.; Gao, C.Y.; Cho, C.H. Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J. Gastroenterol. 2016, 22, 6876–6889. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Shi, D.; Shang, M.; Duan, S.; Guo, L.; Ning, S.; Li, J. Breast cancer cell line phenotype affects sonoporation efficiency under optimal ultrasound microbubble conditions. Med. Sci. Monit. 2018, 24, 9054–9062. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shirai, Y.; Sakamoto, M.; Mori, S.; Kodama, T. Use of a Lymphatic Drug Delivery System and Sonoporation to Target Malignant Metastatic Breast Cancer Cells Proliferating in the Marginal Sinuses. Sci. Rep. 2019, 9, 13242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranjc, S.; Kranjc, M.; Scancar, J.; Jelenc, J.; Sersa, G.; Miklavcic, D. Electrochemotherapy by pulsed electromagnetic field treatment (PEMF) in mouse melanoma B16F10 in vivo. Radiol. Oncol. 2016, 50, 39–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolong, A.; Davalos, R.V.; Rubinsky, B. History of Electroporation. In Irreversible Electroporation in Clinical Practice; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 13–37. ISBN 978-3-319-55113-5. [Google Scholar]
- Petrini, M.; Mattii, L.; Sabbatini, A.; Carulli, G.; Grassi, B.; Cadossi, R.; Ronca, G.; Conte, A. Multidrug resistance and electromagnetic fields. Electromagn. Biol. Med. 1990, 9, 209–212. [Google Scholar] [CrossRef]
- Pickering, S.A.W.; Bayston, R.; Scammell, B.E. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J. Bone Jt. Surg. Ser. B 2003, 85, 588–593. [Google Scholar] [CrossRef] [Green Version]
- Segatore, B.; Setacci, D.; Bennato, F.; Cardigno, R.; Amicosante, G.; Iorio, R. Evaluations of the effects of extremely low-frequency electromagnetic fields on growth and antibiotic susceptibility of escherichia coli and pseudomonas aeruginosa. Int. J. Microbiol. 2012, 2012, 587293. [Google Scholar] [CrossRef] [Green Version]
- Saczko, J.; Pilat, J.; Choromanska, A.; Rembialkowska, N.; Bar, J.; Kaminska, I.; Zalewski, J.; Kulbacka, J. The effectiveness of chemotherapy and electrochemotherapy on ovarian cell lines in vitro. Neoplasma 2016, 63, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Campana, L.G.; Edhemovic, I.; Soden, D.; Perrone, A.M.; Scarpa, M.; Campanacci, L.; Cemazar, M.; Valpione, S.; Miklavčič, D.; Mocellin, S.; et al. Electrochemotherapy-Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur. J. Surg. Oncol. 2019, 45, 92–102. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Reberšek, M.; Vernier, P.T.; Mali, B.; Miklavčič, D. Effects of high voltage nanosecond electric pulses on eucaryotic cells (in vitro): A systematic review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gianulis, E.C.; Labib, C.; Saulis, G.; Novickij, V.; Pakhomova, O.N.; Pakhomov, A.G. Selective susceptibility to nanosecond pulsed electric field (nsPEF) across different human cell types. Cell. Mol. Life Sci. 2016, 74, 1741–1754. [Google Scholar] [CrossRef]
- Romeo, S.; Sannino, A.; Scarfì, M.R.; Vernier, P.T.; Cadossi, R.; Gehl, J.; Zeni, O. ESOPE-Equivalent Pulsing Protocols for Calcium Electroporation: An In Vitro Optimization Study on 2 Cancer Cell Models. Technol. Cancer Res. Treat. 2018, 17, 1533033818788072. [Google Scholar] [CrossRef] [Green Version]
- Frandsen, S.K.; Gissel, H.; Hojman, P.; Eriksen, J.; Gehl, J. Calcium electroporation in three cell lines: A comparison of bleomycin and calcium, calcium compounds, and pulsing conditions. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1204–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Cemazar, M.; Miklavcic, D.; Mir, L.M.; Belehradek, J.; Bonnay, M.; Fourcault, D.; Sersa, G. Electrochemotherapy of tumours resistant to cisplatin: A study in a murine tumour model. Eur. J. Cancer 2001, 37, 1166–1172. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Grandi, M.; Geroni, C.; Giuliani, F.C. Isolation and characterization of a human colon adenocarcinoma cell line resistant to doxorubicin. Br. J. Cancer 1986, 54, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, G.; Bassett, J.; Denney, R. Determination of Calcium and Magnesium. In Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 1989; pp. 328–330. [Google Scholar]
- Jeffery, G.; Bassett, J.; Denney, R. Complexation Titrations. In Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1989; pp. 309–322. [Google Scholar]
- Harris, D.C.; Lucy, C.A. EDTA Titrations. In Daniel C. Harris: Quantitative Chemical Analysis, 9th ed.; W. H. Freeman: New York, NY, USA, 2015; pp. 265–286. [Google Scholar]
- Novickij, V.; Rembialkowska, N.; Staigvila, G.; Kulbacka, J. Effects of extracellular medium conductivity on cell response in the context of sub-microsecond range calcium electroporation. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Forster, S.; Thumser, A.E.; Hood, S.R.; Plant, N. Characterization of rhodamine-123 as a tracer dye for use in in vitro drug transport assays. PLoS ONE 2012, 7, e33253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, H.; Szyjka, A.; Gasiorowski, K. Chemopreventive activity of celastrol in drug-resistant human colon carcinoma cell cultures. Oncotarget 2018, 9, 21211–21223. [Google Scholar] [CrossRef] [Green Version]
- Adkins, C.E.; Mittapalli, R.K.; Manda, V.K.; Nounou, M.I.; Mohammad, A.S.; Terrell, T.B.; Bohn, K.A.; Yasemin, C.; Grothe, T.R.; Lockman, J.A.; et al. P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front. Pharmacol. 2013, 4, 136. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hao, D.C.; Stein, W.D.; Yang, L. A kinetic study of Rhodamine123 pumping by P-glycoprotein. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1671–1676. [Google Scholar] [CrossRef] [Green Version]
- Twentyman, P.R.; Rhodes, T.; Rayner, S. A comparison of rhodamine 123 accumulation and efflux in cells with P-glycoprotein-mediated and MRP-associated multidrug resistance phenotypes. Eur. J. Cancer 1994, 30, 1360–1369. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A comprehensive review of calcium electroporation—A novel cancer treatment modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef] [Green Version]
- Zielichowska, A.; Daczewska, M.; Saczko, J.; Michel, O.; Kulbacka, J. Applications of calcium electroporation to effective apoptosis induction in fibrosarcoma cells and stimulation of normal muscle cells. Bioelectrochemistry 2016, 109, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Lastauskiene, E.; Novickij, V.; Zinkevičiene, A.; Girkontaite, I.; Paškevičius, A.; Švediene, J.; Markovskaja, S.; Novickij, J. Application of pulsed electric fields for the elimination of highly drug-resistant Candida grown under modelled microgravity conditions. Int. J. Astrobiol. 2019, 18, 405–411. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Wu, S.; Feng, H.; Sun, S.; Pan, J.; Zhang, J.; Beebe, S.J. Synergistic Effects of Nanosecond Pulsed Electric Fields Combined with Low Concentration of Gemcitabine on Human Oral Squamous Cell Carcinoma In Vitro. PLoS ONE 2012, 7, e43213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunjachan, S.; Rychlik, B.; Storm, G.; Kiessling, F.; Lammers, T. Multidrug resistance: Physiological principles and nanomedical solutions. Adv. Drug Deliv. Rev. 2013, 65, 1852–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozben, T. Mechanisms and strategies to overcome multiple drug resistance in cancer. FEBS Lett. 2006, 580, 2903–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choromańska, A.; Chwiłkowska, A.; Kulbacka, J.; Baczyńska, D.; Rembiałkowska, N.; Szewczyk, A.; Michel, O.; Gajewska-Naryniecka, A.; Przystupski, D.; Saczko, J. Modifications of Plasma Membrane Organization in Cancer Cells for Targeted Therapy. Molecules 2021, 26, 1850. [Google Scholar] [CrossRef]
- Agarwal, A.; Zudans, I.; Weber, E.A.; Olofsson, J.; Orwar, O.; Weber, S.G. Effect of cell size and shape on single-cell electroporation. Anal. Chem. 2007, 79, 3589–3596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henslee, B.E.; Morss, A.; Hu, X.; Lafyatis, G.P.; Lee, L.J. Electroporation dependence on cell size: Optical tweezers study. Anal. Chem. 2011, 83, 3998–4003. [Google Scholar] [CrossRef] [PubMed]
- Kulbacka, J.; Daczewska, M.; Dubińska-Magiera, M.; Choromańska, A.; Rembiałkowska, N.; Surowiak, P.; Kulbacki, M.; Kotulska, M.; Saczko, J. Doxorubicin delivery enhanced by electroporation to gastrointestinal adenocarcinoma cells with P-gp overexpression. Bioelectrochemistry 2014, 100, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Weżgowiec, J.; Kulbacka, J.; Saczko, J.; Rossowska, J.; Chodaczek, G.; Kotulska, M. Biological effects in photodynamic treatment combined with electropermeabilization in wild and drug resistant breast cancer cells. Bioelectrochemistry 2018, 123, 9–18. [Google Scholar] [CrossRef]
- Hoejholt, K.L.; Mužić, T.; Jensen, S.D.; Dalgaard, L.T.; Bilgin, M.; Nylandsted, J.; Heimburg, T.; Frandsen, S.K.; Gehl, J. Calcium electroporation and electrochemotherapy for cancer treatment: Importance of cell membrane composition investigated by lipidomics, calorimetry and in vitro efficacy. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Levine, Z.A.; Vernier, P.T. Calcium and phosphatidylserine inhibit lipid electropore formation and reduce pore lifetime. J. Membr. Biol. 2012, 245, 599–610. [Google Scholar] [CrossRef]
- Boettcher, J.M.; Davis-Harrison, R.L.; Clay, M.C.; Nieuwkoop, A.J.; Ohkubo, Y.Z.; Tajkhorshid, E.; Morrissey, J.H.; Rienstra, C.M. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry 2011, 50, 2264–2273. [Google Scholar] [CrossRef] [Green Version]
- Pakhomova, O.N.; Gregory, B.; Semenov, I.; Pakhomov, A.G. Calcium-mediated pore expansion and cell death following nanoelectroporation. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2547–2554. [Google Scholar] [CrossRef] [Green Version]
- Cemazar, I.M.; Sersa, G.; Ycld, D.M. Electrochemotherapy with Cisplatin in the Treatment of Tumor Cells Resistant to Cisplatin. Anticancer Res. 1998, 18, 4463–4466. [Google Scholar] [PubMed]
- Condello, M.; D’Avack, G.; Spugnini, E.P.; Meschini, S. View of Electroporation: New strategy to improve the drug uptake and overcome the tumour resistance. Microscopie 2019, 30, 39–43. [Google Scholar]
- Semenov, I.; Xiao, S.; Pakhomova, O.N.; Pakhomov, A.G. Recruitment of the intracellular Ca2+ by ultrashort electric stimuli: The impact of pulse duration. Cell Calcium 2013, 54, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napotnik, T.B.; Wu, Y.H.; Gundersen, M.A.; Miklavčič, D.; Vernier, P.T. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics 2012, 33, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Zharkova, L.P.; Romanchenko, I.V.; Buldakov, M.A.; Priputnev, P.V.; Bolshakov, M.A.; Rostov, V.V. Mitochondrial Membrane Permeability after Nanosecond Electromagnetic Pulsed Exposure. In Proceedings of the 2018 20th International Symposium on High-Current Electronics, ISHCE 2018, Tomsk, Russia, 16–22 September 2018; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018; p. 220. [Google Scholar]
- Vernier, P.T.; Sun, Y.; Gundersen, M.A. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]








| Sample | Ca2+ & Mg2+ [mmol/L] | Ca2+ [mmol/L] | Mg2+ (= [Ca, Mg]−[Ca]) [mmol/L] |
|---|---|---|---|
| 10 mM HEPES | 0.017 ± 0.005 | 0.015 ± 0.006 | 0.00 |
| 10 mM HEPES + sucrose + 1 mmol/L Mg2+ | 0.96 ± 0.03 | 0.010 ± 0.009 | 0.95 ± 0.04 |
| 10 mM HEPES + sucrose + 1 mmol/L Mg2+ + 2 mmol/L Ca2+ | 2.64 ± 0.05 | 1.68 ± 0.02 | 0.96 ± 0.07 |
| Milli-Q | 0.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulbacka, J.; Rembiałkowska, N.; Szewczyk, A.; Moreira, H.; Szyjka, A.; Girkontaitė, I.; Grela, K.P.; Novickij, V. The Impact of Extracellular Ca2+ and Nanosecond Electric Pulses on Sensitive and Drug-Resistant Human Breast and Colon Cancer Cells. Cancers 2021, 13, 3216. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13133216
Kulbacka J, Rembiałkowska N, Szewczyk A, Moreira H, Szyjka A, Girkontaitė I, Grela KP, Novickij V. The Impact of Extracellular Ca2+ and Nanosecond Electric Pulses on Sensitive and Drug-Resistant Human Breast and Colon Cancer Cells. Cancers. 2021; 13(13):3216. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13133216
Chicago/Turabian StyleKulbacka, Julita, Nina Rembiałkowska, Anna Szewczyk, Helena Moreira, Anna Szyjka, Irutė Girkontaitė, Kamil P. Grela, and Vitalij Novickij. 2021. "The Impact of Extracellular Ca2+ and Nanosecond Electric Pulses on Sensitive and Drug-Resistant Human Breast and Colon Cancer Cells" Cancers 13, no. 13: 3216. https://0-doi-org.brum.beds.ac.uk/10.3390/cancers13133216