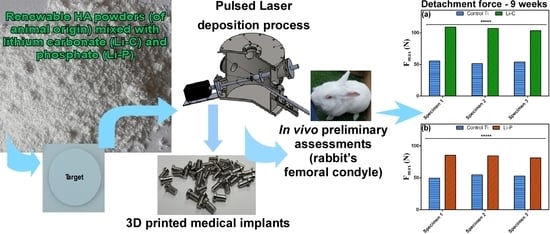
In Vivo Assessment of Bone Enhancement in the Case of 3D-Printed Implants Functionalized with Lithium-Doped Biological-Derived Hydroxyapatite Coatings: A Preliminary Study on Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Printing of Metallic Implants
2.2. Pulsed Laser Deposition (PLD) Experiments
2.2.1. Powders
2.2.2. Target Preparation
2.2.3. Coating Fabrication
2.2.4. Thermal Treatments
2.3. Animals and Surgical Experimental Protocol
2.4. Characterization of Control and Functionalized Three-Dimensional (3D) Ti Implants
2.4.1. Computed Tomography
2.4.2. Mechanical Testing
2.4.3. Scanning Electron Microscopy (SEM)
2.5. Statistical Analysis
3. Results
3.1. Clinical Observations Following Implantation
3.2. Mechanical Testing
3.3. SEM
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Popa, A.C.; Stan, G.E.; Besleaga, C.; Ion, L.; Maraloiu, V.A.; Tulyaganov, D.U.; Ferreira, J.M.F. Submicrometer Hollow Bioglass Cones Deposited by Radio Frequency Magnetron Sputtering: Formation Mechanism, Properties, and Prospective Biomedical Applications. ACS Appl. Mater. Interfaces 2016, 8, 4357–4367. [Google Scholar] [CrossRef] [PubMed]
- Stan, G.E.; Popescu, A.C.; Mihailescu, I.N.; Marcov, D.A.; Mustata, R.C.; Sima, L.E.; Petrescu, S.M.; Ianculescu, A.; Trusca, R.; Morosanu, C.O. On the bioactivity of adherent bioglass thin films synthesized by magnetron sputtering techniques. Thin Solid Films 2010, 518, 5955–5964. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on Ionic Substitutions in Hydroxyapatite Thin Films: Towards Complete Biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef] [Green Version]
- Oladele, I.O.; Agbabiaka, O.; Olasunkanmi, O.G.; Balogun, A.O.; Popoola, M.O. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018, 5, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [Green Version]
- Ballini, A.; Mastrangelo, F.; Gastaldi, G.; Tettamanti, L.; Bukvic, N.; Cantore, S.; Cocco, T.; Saini, R.; Desiate, A.; Gherlone, E.; et al. Osteogenic differentiation and gene expression of dental pulp stem cells under low-level laser irradiation: A good promise for tissue engineering. J. Biol. Regul. Homeost. Agents 2015, 29, 813–822. [Google Scholar] [PubMed]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing Series in Biomaterials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 143–157. [Google Scholar] [CrossRef]
- Barrere, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.; Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium phosphate–based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic, and multiphasic calcium orthophosphates. In Advanced Ceramic Materials; Tiwari, A., Gerhardt, R.A., Szutkowska, M., Eds.; Wiley, Scrivener Publishing: Austin, TX, USA, 2016; pp. 33–95. [Google Scholar] [CrossRef]
- Crespi, R.; Capparè, P.; Gherlone, E. Comparison of magnesium-enriched hydroxyapatite and porcine bone in human extraction socket healing: A histologic and histomorphometric evaluation. Int. J. Oral. Maxillofac. Implant. 2011, 26, 1057–1062. [Google Scholar] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Magnesium-enriched hydroxyapatite compared to calcium sulfate in the healing of human extraction sockets: Radiographic and histomorphometric evaluation at 3 months. J. Periodontol. 2009, 80, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. A Prospective Longitudinal Study on Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients with 1-Year Follow-Up: The Role of CD4+ Level, Smoking Habits, and Oral Hygiene. Clin. Implant Dent. Relat. Res. 2016, 18, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Pesce, V.; Speciale, D.; Sammarco, G.; Patella, S.; Spinarelli, A.; Patella, V. Surgical approach to bone healing in osteoporosis. Clin. Cases Miner. Bone Metab. 2009, 6, 131–135. [Google Scholar] [PubMed]
- León, B.; John, J. Thin Calcium Phosphate Coatings for Medical Implants; Springer-Verlag: New York, NY, USA, 2009; pp. 1–328. [Google Scholar] [CrossRef]
- Available online: https://www.eos.info/en/3d-printing-examples-applications/people-health/medical-3d-printing (accessed on 9 October 2020).
- Jiaxiang, Z.; Anh, Q.V.; Xin, F.; Suresh, B.; Michael, A.R. Pharmaceutical Additive Manufacturing: A Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech 2018, 19, 3388–3402. [Google Scholar] [CrossRef]
- Sima, L.E.; Stan, G.E.; Morosanu, C.O.; Melinescu, A.; Ianculescu, A.; Melinte, R.; Neamtu, J.; Petrescu, S.M. Differentiation of mesenchymal stem cells onto highly adherent radio frequency-sputtered carbonated hydroxylapatite thin films. J. Biomed. Mater. Res. A 2010, 95, 1203–1214. [Google Scholar] [CrossRef]
- Visan, A.; Grossin, D.; Stefan, N.; Duta, L.; Miroiu, F.M.; Stan, G.E.; Sopronyi, M.; Luculescu, C.; Freche, M.; Marsan, O.; et al. Biomimetic nanocrystalline apatite coatings synthesized by Matrix Assisted Pulsed Laser Evaporation for medical applications. Mater. Sci. Eng. B Adv. 2014, 181, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Graziani, G.; Bianchi, M.; Sassoni, E.; Russo, A.; Marcacci, M. Ion-substituted calcium phosphate coatings deposited by plasma-assisted techniques: A review. Mater. Sci. Eng. C 2017, 74, 219–229. [Google Scholar] [CrossRef]
- Vranceanu, D.M.; Ionescu, I.C.; Ungureanu, E.; Cojocaru, M.O.; Vladescu, A.; Cotrut, C.M. Magnesium Doped Hydroxyapatite-Based Coatings Obtained by Pulsed Galvanostatic Electrochemical Deposition with Adjustable Electrochemical Behavior. Coatings 2020, 10, 727. [Google Scholar] [CrossRef]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for bone integrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Asri, R.I.; Harun, W.S.; Hassan, M.A.; Ghani, S.A.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Kim, D.H.; Kovacik, P.; Sojoudi, H.; Wang, M.; Gleason, K.K. Polymer thin films and surface modification by chemical vapor deposition: Recent progress. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 373–393. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, G.; Umar, A. Pulse laser deposited nanostructured ZnO thin films: A review. J. Nanosci. Nanotechnol. 2014, 14, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite. Int. J. Mol. Sci. 2018, 19, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duta, L.; Popescu, A.C. Current Status on Pulsed Laser Deposition of Coatings from Animal-Origin Calcium Phosphate Sources. Coatings 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Chen, C.; Wang, D.; Ji, Q.; Lei, T. Pulsed laser deposition and its current research status in preparing hydroxyapatite thin films. Appl. Surf. Sci. 2005, 252, 1538–1544. [Google Scholar] [CrossRef]
- Kuzanyan, A.S.; Kuzanyan, A.A. Pulsed Laser Deposition of Large-Area Thin Films and Coatings. In Applications of Laser Ablation-Thin Film Deposition, Nanomaterial Synthesis and Surface Modification; Yang, D., Ed.; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef] [Green Version]
- Greer, J.A. History and current status of commercial pulsed laser deposition equipment. J. Phys. D Appl. Phys. 2014, 47, 034005. [Google Scholar] [CrossRef] [Green Version]
- Popescu, A.C.; Florian, P.E.; Stan, G.E.; Popescu-Pelin, G.; Zgura, I.; Enculescu, M.; Oktar, F.N.; Trusca, R.; Sima, L.E.; Roseanu, A.; et al. Physical-chemical characterization and biological assessment of simple and lithium-doped biological-derived hydroxyapatite thin films for a new generation of metallic implants. Appl. Surf. Sci. 2018, 439, 724–735. [Google Scholar] [CrossRef]
- Florian, P.E.; Duta, L.; Grumezescu, V.; Popescu-Pelin, G.; Popescu, A.C.; Oktar, F.N.; Evans, R.W.; Constantinescu, A.R. Lithium-Doped Biological-Derived Hydroxyapatite Coatings Sustain In Vitro Differentiation of Human Primary Mesenchymal Stem Cells to Osteoblasts. Coatings 2019, 9, 781. [Google Scholar] [CrossRef] [Green Version]
- Duta, L.; Chifiriuc, M.C.; Popescu-Pelin, G.; Bleotu, C.; Gradisteanu, G.P.; Anastasescu, M.; Achim, A.; Popescu, A. Pulsed Laser Deposited Biocompatible Lithium-Doped Hydroxyapatite Coatings with Antimicrobial Activity. Coatings 2019, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Duta, L.; Oktar, F.N.; Stan, G.E.; Popescu-Pelin, G.; Serban, N.; Luculescu, C.; Mihailescu, I.N. Novel doped hydroxyapatite thin films obtained by pulsed laser deposition. Appl. Surf. Sci. 2013, 265, 41–49. [Google Scholar] [CrossRef]
- Duta, L.; Mihailescu, N.; Popescu, A.C.; Luculescu, C.R.; Mihailescu, I.N.; Cetin, G.; Gunduz, O.; Oktar, F.N.; Popa, A.C.; Kuncser, A.; et al. Comparative physical, chemical and biological assessment of simple and titanium-doped ovine dentine-derived hydroxyapatite coatings fabricated by pulsed laser deposition. Appl. Surf. Sci. 2017, 413, 129–139. [Google Scholar] [CrossRef]
- Eisenhart, S. EU Regulation 722. In New EU Animal Tissue Regulations in Effect for Some Medical Devices; Emergo: Hong Kong, China, 2013; Available online: https://www.emergobyul.com/blog/2013/09/new-euanimaltissue-regulations-effect-some-medical-devices (accessed on 11 September 2020).
- ISO 22442-1. Medical Devices Utilizing Animal Tissues and Their Derivatives—Part 1: Application of Risk Management; International Organization for Standardization: Berlin, Germany, 2015. [Google Scholar]
- Chioibasu, D.; Mihai, S.-A.; Duta, L.; Popescu, A.C. Additive Manufacturing of Fixing Devices for Metallic Implants by “Laser Melting Deposition” Method. status (unpublished Patent application, A00214, submitted to State Office for Inventions and Trademarks, OSIM, 22 April 2020).
- Lu, Y.P.; Chen, Y.M.; Li, S.T.; Wang, J.H. Surface nanocrystallization of hydroxyapatite coating. Acta Biomater. 2008, 4, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Neyt, J.G.; Buckwalter, J.A.; Carroll, N.C. Use of animal models in musculoskeletal research. Iowa Orthop. J. 1998, 18, 118–123. [Google Scholar]
- Gilsanz, V.; Roe, T.F.; Gibbens, D.T.; Schulz, E.E.; Carlson, M.E.; Gonzalez, O.; Boechat, M.I. Effect of sex steroids on peak bone density of growing rabbits. Am. J. Physiol. 1988, 255, E416–E421. [Google Scholar] [CrossRef]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Sun, J.-F.; Chu, P.K.; Han, Y.; Zhang, Y.-M. Bone integration capability of a series of strontium-containing hydroxyapatite coatings formed by micro-arc oxidation. J. Biomed. Mater. Res. A 2013, 101, 2465–2480. [Google Scholar] [CrossRef]
- Eason, R. Pulsed Laser Deposition of Thin FILMS: Applications-Led Growth of Functional Materials, 1st ed.; Wiley & Sons Interscience: Hoboken, NY, USA, 2007; pp. 1–705. [Google Scholar]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Goller, G.; Oktar, F.N.; Agathopoulos, S.; Tulyaganov, D.U.; Ferreira, J.M.F.; Kayali, E.S.; Peker, I. Effect of sintering temperature on mechanical and microstructural properties of bovine hydroxyapatite (BHA). J. Sol-Gel Sci. Techn. 2006, 37, 111–115. [Google Scholar] [CrossRef]
- Sun, R.-X.; Lv, Y.; Niu, Y.-R.; Zhao, X.-H.; Cao, D.-S.; Tang, J.; Sun, X.-C.; Chen, K.-Z. Physicochemical and biological properties of bovine-derived porous hydroxyapatite/collagen composite and its hydroxyapatite powders. Ceram. Int. 2017, 43, 16792–16798. [Google Scholar] [CrossRef]
- Rahavi, S.S.; Ghaderi, O.; Monshi, A.; Fathi, M.H. A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ. J. Non-Ferrous Metals 2017, 58, 276–286. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Rajendran, A.; Adeogun, A.I.; Idowu, M.A.; Kareem, S.O.; Pattanayak, D.K. Synthesis of organic derived hydroxyapatite scaffold from pig bone waste for tissue engineering applications. Adv. Powder Technol. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.-X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.; Albrektsson, T. A novel in vivo method for quantifying the interfacial biochemical bond strength of bone implants. J. R Soc. Interface 2009, 7, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, F.J.; Cottrell, J.M.; Deng, X.-H.; Crowder, K.N.; Doty, S.B.; Avaltroni, M.J.; Warren, R.F.; Wright, T.M.; Schwartz, J. A novel surface treatment for porous metallic implants that improves the rate of bony ongrowth. J. Biomed. Mater. Res. A 2008, 86, 857–864. [Google Scholar] [CrossRef]
- Schumacher, T.C.; Tushtev, K.; Wagner, U.; Becker, C.; Große Holthaus, M.; Hein, S.B.; Haack, J.; Engelhardt, C.H.M.; Khassawna, T.E.; Rezwan, K. A novel, hydroxyapatite-based screw-like device for anterior cruciate ligament (ACL) reconstructions. Knee 2017, 24, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Aparicioa, C.; Padrósb, A.; Gil, F.-J. In vivo evaluation of micro-rough and bioactive titanium dental implants using histometry and pull-out tests. J. Mech. Behav. Biomed. 2011, 4, 1672–1682. [Google Scholar] [CrossRef]
- Kumar, R.R.; Maruno, S. Functionally graded coatings of HA–G–Ti composites and their in vivo studies. Mater. Sci. Eng. A Struct. 2002, 334, 156–162. [Google Scholar] [CrossRef]
- Hayami, T.; Hontsu, S.; Higuchi, Y.; Nishikawa, H.; Kusunoki, M. Osteoconduction of a stoichiometric and bovine hydroxyapatite bilayer-coated implant, hydroxyapatite bilayer-coated implant. Clin. Oral Implant. Res. 2011, 22, 774–776. [Google Scholar] [CrossRef]
- Zhang, K.; Alaohali, A.; Sawangboon, N.; Sharpe, P.T.; Brauer, D.S.; Gentleman, E. A comparison of lithium-substituted phosphate and borate bioactive glasses for mineralised tissue repair. Dent. Mater. 2019, 35, 919–927. [Google Scholar] [CrossRef]
- Khan, P.K.; Mahato, A.; Kundu, B.; Nandi, S.K.; Mukherjee, P.; Datta, S.; Sarkar, S.; Mukherjee, J.; Nath, S.; Balla, V.K.; et al. Influence of single and binary doping of strontium and lithium on in vivo biological properties of bioactive glass scaffolds. Sci. Rep. UK 2016, 6, 32964. [Google Scholar] [CrossRef] [Green Version]
- Zamani, A.; Omrani, G.R.; Nasab, M.M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Cohen, O.; Rais, T.; Lepkifker, E.; Vered, I. Lithium Carbonate Therapy is not a Risk Factor for Osteoporosis. Horm. Metab. Res. 1998, 30, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implant. 2011, 26, 866–872. [Google Scholar] [PubMed]










| Implant Type | Osseous Density (Mean ± SD) [HU] | |
|---|---|---|
| 4 Weeks | 9 Weeks | |
| Control (Ti) | 811 ± 21 | 850 ± 57 |
| Li-C | 1068 ± 70 | 1156 ± 40 |
| Implant Type | Osseous Density (Mean ± SD) [HU] | |
|---|---|---|
| 4 Weeks | 9 Weeks | |
| Control (Ti) | 818 ± 36 | 855 ± 61 |
| Li-P | 1053 ± 40 | 1172 ± 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, L.; Neamtu, J.; Melinte, R.P.; Zureigat, O.A.; Popescu-Pelin, G.; Chioibasu, D.; Oktar, F.N.; Popescu, A.C. In Vivo Assessment of Bone Enhancement in the Case of 3D-Printed Implants Functionalized with Lithium-Doped Biological-Derived Hydroxyapatite Coatings: A Preliminary Study on Rabbits. Coatings 2020, 10, 992. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings10100992
Duta L, Neamtu J, Melinte RP, Zureigat OA, Popescu-Pelin G, Chioibasu D, Oktar FN, Popescu AC. In Vivo Assessment of Bone Enhancement in the Case of 3D-Printed Implants Functionalized with Lithium-Doped Biological-Derived Hydroxyapatite Coatings: A Preliminary Study on Rabbits. Coatings. 2020; 10(10):992. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings10100992
Chicago/Turabian StyleDuta, Liviu, Johny Neamtu, Razvan P. Melinte, Oana A. Zureigat, Gianina Popescu-Pelin, Diana Chioibasu, Faik N. Oktar, and Andrei C. Popescu. 2020. "In Vivo Assessment of Bone Enhancement in the Case of 3D-Printed Implants Functionalized with Lithium-Doped Biological-Derived Hydroxyapatite Coatings: A Preliminary Study on Rabbits" Coatings 10, no. 10: 992. https://0-doi-org.brum.beds.ac.uk/10.3390/coatings10100992