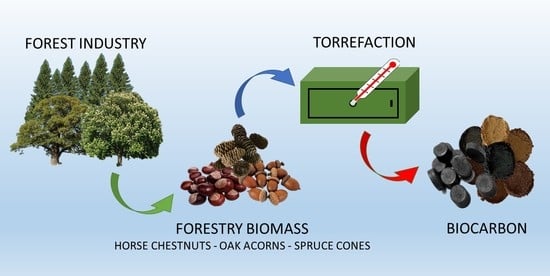
Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used in the Research
2.2. Samples Preparation and Torrefaction Procedure
2.3. Proximate Analysis
2.4. Additional Analysis
3. Results and Discussion
3.1. Results of the Proximate Analysis
3.2. Results of Additional Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AC | ash content |
| DSC | differential scanning calorimetry |
| DTG | derivative thermogravimetry |
| EDR | energy densification ratio |
| EU | European Union |
| EY | energy yield |
| FCC | fixed carbon content |
| HHV | higher heating value |
| MC | moisture content |
| MY | mass yield |
| RES | renewable energy sources |
| TGA | thermogravimetric analysis |
| WDPT | water drop penetration time |
| VMC | volatile matter content |
| VOC | volatile organic compound |
| ρB | bulk density |
| ρS | specific density |
| ε | porosity |
References
- International Energy Agency. Data and Statistics: Energy Consumption, Electricity Final Consumption; World. IEA: Paris, France, 2019. [Google Scholar]
- Hawkins, E.; Ortega, P.; Suckling, E.; Schurer, A.; Hegerl, G.; Jones, P.; van Oldenborgh, G.J. Estimating Changes in Global Temperature since the Preindustrial Period. Bull. Am. Meteorol. Soc. 2017, 98, 1841–1856. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Intergovernmental Panel on Climate Change. Special Report: Global Warming of 1.5 °C; IPCC: Saint-Aubin, France, 2018. [Google Scholar]
- Hamelin, L.; Borzęcka, M.; Kozak, M.; Pudełko, R. A spatial approach to bioeconomy: Quantifying the residual biomass potential in the EU-27. Renew. Sustain. Energy Rev. 2019, 100, 127–142. [Google Scholar] [CrossRef]
- Toklu, E. Biomass Energy potential and utilization in Turkey. Renew. Energy 2017, 107, 235–244. [Google Scholar] [CrossRef]
- Dyjakon, A.; Garcia-Galindo, D. Implementing Agricultural Pruning to Energy in Europe: Technical, Economic and Implementation Potentials. Energies 2019, 12, 1513. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, R.F. Forest Products Utilization within a Circular Bioeconomy. For. Prod. J. 2020, 70, 4–9. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Smędzik, M. The Influence of Torrefaction Temperature on Hydrophobic Properties of Waste Biomass from Food Processing. Energies 2019, 12, 4609. [Google Scholar] [CrossRef] [Green Version]
- Tabakaev, R.; Shanenkov, I.; Kazakov, A.; Zavorin, A. Thermal processing of biomass into high-calorific solid composite fuel. J. Anal. Appl. Pyrol. 2017, 124, 94–102. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Greenhouse Gas Balances of Bioenergy Systems; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-008-10-1036-5. [Google Scholar]
- Qiaoming, L.; Chmely, S.C.; Abdoulmoumine, N. Biomass Treatment Strategies for Thermochemical Conversion. Energy Fuels 2017, 31, 3525–3536. [Google Scholar] [CrossRef]
- Loehle, C. Carbon Sequestration Due to Commercial Forestry: An Equilibrium Analysis. Forest Prod. J. 2020, 60–63. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M. A new approach for evaluating biochar quality from Virginia Mallow biomass thermal processing. J. Clean. Prod. 2019, 214, 356–364. [Google Scholar] [CrossRef]
- Chen, D.; Mei, J.; Li, H.; Li, Y.; Lu, M.; Ma, T.; Ma, Z. Combined pretreatment with torrefaction and washing using torrefaction liquid products to yield upgraded biomass and pyrolysis products. Bioresour. Technol. 2017, 228, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Stępień, P.; Pulka, J.; Białowiec, A. Organic Waste Torrefaction–A Review: Reactor Systems, and the Biochar Properties. In Pyrolysis; IntechOpen: London, UK, 2017; ISBN 978-953-51-3312-4. [Google Scholar]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wand, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mahyoub, S.A.A.; Liao, W.; Xia, S.; Zhao, H.; Guo, M.; Ma, P. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour. Technol. 2017, 223, 20–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, K. Thermal and chemical characteristics of torrefied biomass derived from a generated volatile atmosphere. Energy 2018, 165, 235–245. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Czerep, M.; Kudlek, E.; Wnukowski, M.; Yang, W. Torrefaction of wheat-barley straw: Composition and toxicity of torrefaction condensates. Biomass Bioenergy 2019, 129, 105335. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, S.; Liu, L.; Xu, D.; Xiong, Y. Investigation of representative components of flue gas used as torrefaction pretreatment atmosphere and its effects on fast pyrolysis behaviors. Bioresour. Technol. 2018, 267, 584–590. [Google Scholar] [CrossRef]
- Li, S.X.; Chen, C.Z.; Li, M.F.; Xiao, X. Torrefaction of corncob to produce charcoal under nitrogen and carbon dioxide atmospheres. Bioresour. Technol. 2018, 249, 348–353. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Bi, X. A State of the Art Review of Biomass Torrefaction, Densification and Application. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Via, B.K.; Adhikari, S.; Taylor, S. Modeling for proximate analysis and heating value of torrefied biomass with vibration spectroscopy. Bioresour. Technol. 2013, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of biomass components in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and product characterization. J. Anal. Appl. Pyrol. 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrol. 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Cardona, S.; Gallego, L.J.; Valencia, V.; Martinez, E.; Rios, L.A. Torrefaction of eucalyptus-tree residues: A new method for energy and mass balances of the process with the best torrefaction conditions. Sustain. Energy Techn. 2019, 31, 17–24. [Google Scholar] [CrossRef]
- Uslu, A.; Faaij, A.P.C.; Bergman, P.C.A. Pre-Treatment Technologies and their Effect on International Bioenergy Supply Chain Logistics. Techno-Economic Evaluation of Torrefaction, Fast Pyrolysis and Palletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Zhang, M.; Zhang, K.; Wang, D.; Lei, C. A fundamental research on synchronized torrefaction and pelleting of biomass. Renew. Energy 2019, 142, 668–676. [Google Scholar] [CrossRef]
- Acharjee, T.C.; Coronella, C.J.; Vasquez, V.R. Effect of thermal pretreatment on equilibrium moisture content of lignocellulosic biomass. Bioresour. Technol. 2011, 102, 4849–4854. [Google Scholar] [CrossRef]
- Kanwal, S.; Chaudhry, N.; Munir, S.; Sana, H. Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag. 2019, 88, 280–290. [Google Scholar] [CrossRef]
- Pahla, G.; Ntuli, F.; Muzenda, E. Torrefaction of landfill food waste for possible application in biomass co-firing. Waste Manag. 2018, 71, 512–520. [Google Scholar] [CrossRef]
- Colin, B.; Dirion, J.L.; Arlabosse, P.; Salvador, S. Quantification of the torrefaction effects on the grindability and the hygroscopicity of wood chips. Fuel 2017, 197, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, J.G.; Moya, R.; Puente-Urbina, A.; Rodriguez-Zuniga, A. Thermogravimetric, Volatilization Rate, and Differential Scanning Calorimetry Analyses of Biomass of Tropical Plantation Species of Costa Rica Torrefied at Different Temperatures and Times. Energies 2018, 11, 696. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Opportunities for Utilization of Non-Conventional Energy Sources for Biomass Pretreatment. Bioresour. Technol. 2016, 199, 398–407. [Google Scholar] [CrossRef] [PubMed]
- PN-EN ISO 18134-2:2017-03E. Solid Biofuels. Determination of Moisture Content—Oven Dry Method—Part 2: Total Moisture—Simplified Method; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- PN-EN ISO 18122:2015. Solid Biofuels. Determination of Ash Content; European Committee for Standardization: Brussels, Belgium, 2015. [Google Scholar]
- PN-EN ISO 18123:2016-01. Solid Fuels. Determination of Volatile Content by Gravimetric Method; European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- PN-EN ISO 18125:2017-07. Solid Biofuels. Determination of Calorific Value; European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- ASTM D 3172-73. Standard Method for Proximate Analysis of Coal and Coke; ASTM International: Conshohocken, PA, USA, 1984. [Google Scholar]
- Doerr, S.H. On Standardizing the “Water Drop Penetration Time” and the “Molarity of An Ethanol Droplet” Techniques to Classify Soil Hydrophobicity: A Case Study Using Medium Textured Soils. Earth Surf. Process. Landf. 1998, 23, 663–668. [Google Scholar] [CrossRef]
- Guatam, R.; Ashwath, N. Hydrophobicity of 43 Potting Media: Its Implications for Raising Seedlings in Revegetation Programs. J. Hydrol. 2012, 430–431, 111–117. [Google Scholar] [CrossRef]
- PN-EN 1237:2000. Fertilizers—Determination of Bulk Density (Tapped); European Committee for Standardization: Brussels, Belgium, 2000. [Google Scholar]
- PN-EN 1936:2010. Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity; European Committee for Standardization: Brussels, Belgium, 2010. [Google Scholar]
- Chin, K.L.; H’ng, P.S.; Go, W.Z.; Wong, W.Z.; Lim, T.W.; Maminski, M.; Paridah, M.T.; Luqman, A.C. Optimization of torrefaction conditions for high energy density solid biofuel from oil palm biomass and fast growing species available in Malaysia. Ind. Crops Prod. 2013, 49, 768–774. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Colin, B.; Chang, J.S.; Petrissans, A.; Bi, X.; Petrissans, M. Hygroscopic transformation of woody biomass torrefaction for carbon storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Berther, M.A.; Commandre, J.M.; Rouau, X.; Gontard, N.; Angellier-Coussy, H. Torrefaction Treatment of Lignocellulosic Fibres for Improving Fibre, Matrix Adhesion in a Biocomposite. Mater. Des. 2016, 92, 223–232. [Google Scholar] [CrossRef]
- Wang, Z.; Lim, C.J.; Grace, J.R.; Li, H.; Parise, N.R. Effects of temperature and particle size on biomass torrefaction in a slot-rectangular spouted bed reactor. Bioresour. Technol. 2017, 244, 281–288. [Google Scholar] [CrossRef]
- He, J.; Zhu, L.; Liu, C.; Bai, Q. Optimization of the oil agglomeration for high-ash content coal slime based on design and analysis of response surface methodology (RSM). Fuel 2019, 254, 115560. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. The influence of freezing temperature storage on the mechanical durability of commercial pellets from biomass. Energies 2019, 12, 2627. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Hu, S.; Xiang, J.; Su, S.; Sun, L.-S.; Xu, K.; Yao, Y. Release characteristics of alkali and alkaline earth metallic species during biomass pyrolysis and steam gasification process. Bioresour. Technol. 2012, 116, 278–284. [Google Scholar] [CrossRef]
- Noda, R.; Matsuhisa, Y.; Ito, T.; Horio, M. Alkali metal evolution characteristics of wood biomass during pyrolysis and gasification. In Proceedings of the Annual Conference of The Japan Institute of Energy, Sapporo, Japan, 30–31 July 2003. [Google Scholar]
- Uemura, Y.; Omar, W.N.; Tsutsui, T.; Yusup, S.B. Torrefaction of oil palm wastes. Fuel 2011, 90, 2585–2591. [Google Scholar] [CrossRef]
- Riaza, J.; Gibbins, J.; Chalmers, H. Ignition and combustion of single particles of coal and biomass. Fuel 2017, 202, 650–655. [Google Scholar] [CrossRef]
- Tong, S.; Xiao, L.; Li, X.; Zhu, X.; Liu, H.; Luo, G.; Worasuwannarak, N.; Kerdsuwan, S.; Fungtammasan, B.; Yao, H. A gas-pressurized torrefaction method for biomass wastes. Energy Convers. Manag. 2018, 173, 29–36. [Google Scholar] [CrossRef]
- Correia, R.; Goncalves, M.; Nobre, C.; Mendes, B. Impact of torrefaction and low-temperature carbonization on the properties of biomass wastes from Arundo donax L. and Phoenix canariensis. Bioresour. Technol. 2017, 223, 210–218. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Q.; Lei, J.; Hong, H.; Jin, H. New-solar biomass power generation system integrated a two-stage gasifier. Appl. Energy 2017, 194, 310–319. [Google Scholar] [CrossRef]
- Geng, C.; Yang, W.; Sun, X.; Wang, X.; Bai, Z.; Zhang, X. Emission factors, ozone and secondary organic aerosol formation potential of volatile organic compounds emitted from industrial biomass boilers. Int. J. Environ. Sci. 2019, 83, 64–72. [Google Scholar] [CrossRef]
- Conag, A.T.; Villahermosa, J.E.R.; Cabatingan, L.K.; Go, A.W. Energy densification of sugarcane leaves through torrefaction under minimized oxidative atmosphere. Energy Sustain. Dev. 2018, 42, 160–169. [Google Scholar] [CrossRef]
- Uzun, H.; Yildiz, Z.; Goldfarb, J.L.; Ceylan, S. Improved prediction of higher heating value of biomass using an artificial neural network model based on proximate analysis. Bioresour. Technol. 2017, 234, 122–130. [Google Scholar] [CrossRef]
- Świechowski, K.; Liszewski, M.; Bąbelewski, M.; Koziel, J.A.; Białowiec, A. Fuel properties of torrefied biomass from pruning of oxytree. Data 2019, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Tumuluru, J.S.; Sokhansanj, S.; Wright, C.T.; Boardman, R.D.; Hess, R.J. Review on Biomass Torrefaction Process and Product Properties and Design of Moving Bed Torrefaction System Model Development. In Proceedings of the ASABE Annual International Meeting, Louisville, KY, USA, 7–10 August 2011. [Google Scholar]
- Chen, Y.; Liu, B.; Yang, H.; Yang, Q.; Chen, H. Evolution of Functional Groups and Pore Structure During Cotton and Corn Stalks Torrefaction and its Correlation with Hydrophobicity. Fuel 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Alvarez, A.; Gutierrez, G.; Matos, M.; Pizarro, C.; Bueno, J.L. Torrefaction of short rotation coppice of poplar under oxidative and non-oxidative atmosphere. Multidiscip. Digit. Publ. Inst. Proc. 2018, 2, 1479. [Google Scholar] [CrossRef] [Green Version]
- Pouzet, M.; Dubois, M.; Charlet, K.; Petit, E.; Beakou, A.; Dupont, C. Fluorination/Torrefaction Combination to Further Improve the Hydrophobicity of Wood. Macromol. Chem. Phys. 2019, 220. [Google Scholar] [CrossRef]
- Piccand, M.; Bianchi, S.; Halaburt, E.I.; Mayer, I. Characterization of extractives from biomasses of the alpine forests and their antioxidative efficacy. Ind. Crops. Prod. 2019, 142, 111832. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Han, J.S.; Micales, J.A.; Young, R.A. Decay Resistance in Conifer Seed Cones: Role of Resin Acids as Inhibitors of Decomposition by White-Rot Fungi. Holzforschung 1994, 48, 278–284. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Basu, P.; Acharya, B. A comprehensive Review on Biomass Torrefaction. IJREB 2014. [Google Scholar] [CrossRef]
- Bach, Q.V.; Skreiberg, O. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- Almeida, G.; Brito, J.O.; Perre, P. Alterations in energy properties of eucalyptus wood and bark subjected to torrefaction: The potential of mass loss as a synthetic indicator. Bioresour. Technol. 2010, 101, 9778–9784. [Google Scholar] [CrossRef]
- Poudel, J.; Ohm, T.I.; Oh, S.C. A study on torrefaction of food waste. Fuel 2015, 140, 275–281. [Google Scholar] [CrossRef]
















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyjakon, A.; Noszczyk, T. Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones. Energies 2020, 13, 2468. https://0-doi-org.brum.beds.ac.uk/10.3390/en13102468
Dyjakon A, Noszczyk T. Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones. Energies. 2020; 13(10):2468. https://0-doi-org.brum.beds.ac.uk/10.3390/en13102468
Chicago/Turabian StyleDyjakon, Arkadiusz, and Tomasz Noszczyk. 2020. "Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones" Energies 13, no. 10: 2468. https://0-doi-org.brum.beds.ac.uk/10.3390/en13102468