Technological Effectiveness of Sugar-Industry Effluent Methane Fermentation in a Fluidized Active Filling Reactor (FAF-R)
Abstract
:1. Introduction
2. Materials and Methods
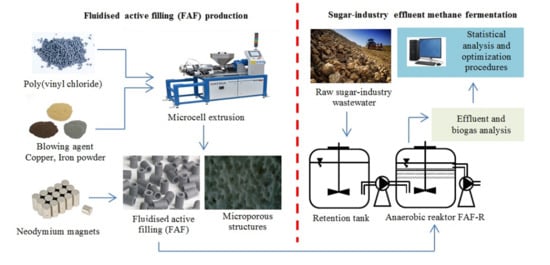
2.1. General Study Design
2.2. Materials
2.2.1. Sugar-Industry Effluent
2.2.2. Anaerobic Sludge
2.2.3. Fluidized Active Filling (FAF)
2.3. Design and Operation of the Process System
2.4. Analytical Methods
2.5. Statistical Analysis and Optimisation Procedures
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FAF | fluidized active filling |
| FAF-R | fluidized active filling reactor |
| OLR | organic load rate (kg COD/m3·d) |
| HRT | hydraulic retention time [day] |
| COD | chemical oxygen demand [mg O2/dm3] |
| BOD5 | biological oxygen demand [mg O2/dm3] |
| FOS/TAC | volatile organic acid and buffer capacity ratio |
References
- Ahmed, E. Assessment of the Liquid and Solid Waste from EL Gunied Sugar Factory. Ph.D. Thesis, University of Khartoum Dspace (UofK), Khartoum, Khartoum State, Sudan, 2015. [Google Scholar]
- Gondudey, S.; Chaudhari, P.K. Treatment of Sugar Industry Effluent Through SBR Followed by Electrocoagulation. Sugar Tech 2020, 22, 303–310. [Google Scholar] [CrossRef]
- Sahu, O. Significance of iron compounds in chemical and electro-oxidation treatment of sugar industry wastewater: Batch reaction. Environ. Qual. Manag. 2019, 29, 113–123. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Kloos, H.; Van Hulle, S.W.H. Physicochemical Properties of the Sugar Industry and Ethanol Distillery Wastewater and Their Impact on the Environment. Sugar Tech 2019, 21, 265–277. [Google Scholar] [CrossRef]
- Farhadian, M.; Borghei, M.; Umrania, V.V. Treatment of beet sugar wastewater by UAFB bioprocess. Bioresour. Technol. 2007, 98, 3080–3083. [Google Scholar] [CrossRef] [PubMed]
- Lettinga, G.; Hulshoff Pol, L.W. UASB-process design for various types of wastewaters. Water Sci. Technol. 1991, 24, 87–107. [Google Scholar] [CrossRef]
- Pradeep, N.V.; Anupama, S.; Kumar, J.M.A.; Vidyashree, K.G.; Lakshmi, P.; Ankitha, K.; Pooja, J. Treatment of sugar industry wastewater in anaerobic downflow stationary fixed film (DSFF) reactor. Sugar Tech 2014, 16, 9–14. [Google Scholar] [CrossRef]
- Hamoda, M.F.; Al-Sharekh, H.A. Sugar wastewater treatment with aerated fixed-film biological systems. Water Sci. Technol. 1999, 40, 313–321. [Google Scholar] [CrossRef]
- Sharma, S.; Simsek, H. Sugar beet industry process wastewater treatment using electrochemical methods and optimization of parameters using response surface methodology. Chemosphere 2020, 238, 124669. [Google Scholar] [CrossRef]
- Fernandez-Rodríguez, J.; Perez, M.; Romero, L.I. Temperature-phased anaer- obic digestion of industrial organic fraction of municipal solid waste: A batch study. Chem. Eng. J. 2015, 270, 597–604. [Google Scholar] [CrossRef]
- Harb, M.; Lou, E.; Smith, A.L.; Stadler, L.B. Perspectives on the fate of micropollutants in mainstream anaerobic wastewater treatment. Curr. Opin. Biotechnol. 2019, 57, 94–100. [Google Scholar] [CrossRef]
- Kong, Z.; Li, L.; Xue, Y.; Yang, M.; Li, Y.-Y. Challenges and prospects for the anaerobic treatment of chemical-industrial organic wastewater: A review. J. Clean. Prod. 2019, 231, 913–927. [Google Scholar] [CrossRef]
- Liu, X.; Li, A.; Ma, L.; Jing, Z.; Yang, J.; Tang, Y.; Hu, B. A comparison on phosphorus release and struvite recovery from waste activated sludge by different treatment methods. Int. Biodeterior. Biodegrad. 2020, 148, 104878. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Yen, H.-W.; Nomanbhay, S.; Ho, Y.-C.; Show, P.L. Transformation of Biomass Waste into Sustainable Organic Fertilizers. Sustainability 2019, 11, 2266. [Google Scholar] [CrossRef] [Green Version]
- Samuel, A.; Bungau, S.; Tit, D.M.; Melinte, E.; Purza, L.; Badea, G. Effects of Long Term Application of Organic and Mineral Fertilizers on Soil Enzymes, Bucharest-Original Edition. Rev. Chim. 2018, 69, 2608–2612. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D. Photosynthesis Assisted Anaerobic Digestion of Cattle Manure Leachate in a Hybrid Bioreactor: An Integrated System for Enhanced Wastewater Treatment and Methane Production. Chem. Eng. J. 2017, 330, 616–624. [Google Scholar] [CrossRef]
- Łagód, G.; Duda, S.M.; Majerek, D.; Szutt, A.; Dołhańczuk-Śródka, A. Application of Electronic Nose for Evaluation of Wastewater Treatment Process Effects at Full-Scale WWTP. Processes 2019, 7, 251. [Google Scholar] [CrossRef] [Green Version]
- González, D.; Guerra, N.; Colón, J.; Gabriel, D.; Ponsá, S.; Sánchez, A. Characterization of the Gaseous and Odour Emissions from the Composting of Conventional Sewage Sludge. Atmosphere 2020, 11, 211. [Google Scholar] [CrossRef] [Green Version]
- Orzi, V.; Cadena, E.; D’Imporzano, G.; Artola, A.; Davoli, E.; Crivelli, M.; Adani, F. Potential odour emission measurement in organic fraction of municipal solid waste during anaerobic digestion: Relationship with process and biological stability parameters. Bioresour. Technol. 2010, 101, 7330–7337. [Google Scholar] [CrossRef]
- Song, X.; Luo, W.; Hai, F.I.; Price, W.E.; Guo, W.; Ngo, H.H.; Nghiem, L.D. Resource recovery from wastewater by anaerobic membrane bioreactors: Opportunities and challenges. Bioresour. Technol. 2018, 270, 669–677. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Varjani, S.; Liu, Y.; Deng, L.; Cheng, C. Selective production of volatile fatty acids at different pH in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 283, 120–128. [Google Scholar] [CrossRef]
- Atelge, R.; Atabani, A.E.; Banu, R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yıldız, Y.; Ünalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Pu, Y.; Huang, J.; Ngo, H.H.; Zeng, Y.; Li, Y. Nutrients removal performance and sludge properties using anaerobic fermentation slurry from food waste as an external carbon source for wastewater treatment. Bioresour. Technol. 2019, 271, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Dębowski, M.; Zieliński, M.; Krzemieniewski, M. Enhancement of Dairy Wastewater Treatment in a Combined Anaerobic Baffled and Biofilm Reactor with Magneto-Active Packing Media. J. Ecol. Eng. 2018, 19, 165–171. [Google Scholar] [CrossRef]
- Petta, L.; De Gisi, S.; Casella, P.; Farina, R.; Notarnicola, M. Evaluation of the treatability of a winery distillery (vinasse) wastewater by UASB, anoxic-aerobic UF-MBR and chemical precipitation/ adsorption. J. Environ. Manag. 2017, 201, 177–189. [Google Scholar] [CrossRef]
- Ojo, P.; Ifelebuegu, A.O. The Effects of Aluminium- and Ferric-Based Chemical Phosphorus Removal on Activated Sludge Digestibility and Dewaterability. Processes 2019, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Kazadi Mbamba, C.; Lindblom, E.; Flores-Alsina, X.; Tait, S.; Anderson, S.; Saagi, R.; Batstone, D.J.; Gernaey, K.V.; Jeppsson, U. Plant-wide model-based analysis of iron dosage strategies for chemical phosphorus removal in wastewater treatment systems. Water Res. 2019, 155, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Yamamoto-Ikemoto, R. Nitrogen and Phosphorus Removal from Wastewater Treatment Plant Effluent via Bacterial Sulfate Reduction in an Anoxic Bioreactor Packed with Wood and Iron. Int. J. Environ. Res. Public Health 2014, 11, 9835–9853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lay, C.H.; Vo, T.P.; Lin, P.Y.; Abdul, P.M.; Liu, C.M.; Lin, C.Y. Anaerobic hydrogen and methane production from low-strength beverage wastewater. Int. J. Hydrogen Energy 2019, 44, 14351–14361. [Google Scholar] [CrossRef]
- Li, B.; Dinkler, K.; Zhao, N.; Sobhi, M.; Merkle, W.; Liu, S.; Dong, R.; Oechsner, H.; Guo, J. Influence of anaerobic digestion on the labile phosphorus in pig, chicken, and dairy manure. Sci. Total Environ. 2020, 737, 140234. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Krzemieniewski, M.; Makowska, M.; Grądkowski, M.; Tor-Świątek, A. Simulated dairy wastewater treatment in a pilot plant scale magneto-active hybrid anaerobic biofilm reactor (MA-HABR). Braz. J. Chem. Eng. 2018, 35, 553–562. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, P.; Fazzino, F.; Folino, A.; Scibetta, S.; Sidari, R. Improvement of semi-continuous anaerobic digestion of pre-treated orange peel waste by the combined use of zero valent iron and granular activated carbon. Biomass Bioenergy 2019, 129, 105337. [Google Scholar] [CrossRef]
- Zhao, B.; Sha, H.; Li, J.; Cao, S.; Wang, G.; Yang, Y. Static magnetic field enhanced methane production via stimulating the growth and composition of microbial community. J. Clean. Prod. 2020, 271, 122664. [Google Scholar] [CrossRef]
- Germec, M.; Demirci, A.; Turhan, I. Biofilm reactors for value-added products production: An in-depth review. Biocatal. Agric. Biotechnol. 2020, 27, 101662. [Google Scholar] [CrossRef]
- Shi, R.; Xu, H.; Zhang, Y. Enhanced treatment of wastewater from the vitamin C biosynthesis industry using a UASB reactor supplemented with zerovalent iron. Environ. Technol. 2011, 32, 1859–1865. [Google Scholar] [CrossRef]
- Lin, H.; King, A.; Williams, N.; Hu, B. Hydrogen sulfide removal via appropriate metal ions dosing in anaerobic digestion. Environ. Prog. Sustain. Energy 2017, 36, 1405–1416. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Y.; Quan, X.; Liu, Y.; Onu, P. A Built-In Zero Valent Iron Anaerobic Reactor to Enhance Treatment of Azo Dye Wastewater. Water Sci. Technol. 2011, 63, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Dębowski, M.; Krzemieniewski, M.; Brudniak, A.; Kisielewska, M. Possibility of improving technological effectiveness of dairy wastewater treatment through application of active fillings and microwave radiation. J. Water Chem. Technol. 2016, 38, 342–348. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Krzemieniewski, M.; Brudniak, A. Effect of magneto-active filling on the effectiveness of methane fermentation of dairy wastewaters. Int. J. Green Energy 2014. [Google Scholar] [CrossRef]
- Nacheva, P.M.; Chávez, G.M.; Chacón, J.M.; Chuil, A.C. Treatment of cane sugar mill wastewater in an upflow anaerobic sludge bed reactor. Water Sci. Technol. 2009, 60, 1347–1352. [Google Scholar] [CrossRef]
- Hampannavar, U.S.; Shivayogimath, C.B. Anaerobic treatment of sugar industry wastewater by Upflow anaerobic sludge blanket reactor at ambient temperature. Int. J. Environ. Sci. 2010, 1, 631–639. [Google Scholar]
- Zhang, L.; Ban, Q.; Li, J.; Wan, C. Functional bacterial and archaeal dynamics dictated by pH stress during sugar refinery wastewater in a UASB. Bioresour. Technol. 2019, 288, 121464. [Google Scholar] [CrossRef] [PubMed]
- Barrera, E.L.; Spanjers, H.; Romero, O.; Rosa, E.; Dewulf, J. A successful strategy for start-up of lab-scale UASB reactor treating sulfate-rich sugar cane vinasse. J. Chem. Technol. Biotechnol. 2019, 95, 205–212. [Google Scholar] [CrossRef]
- Kushwaha, J.P. A review on sugar industry wastewater: Sources, treatment technologies, and reuse. Desalin. Water Treat. 2013, 53, 309–318. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Kloos, H.; Van Hulle, S.W.H. Anaerobic treatment of blended sugar industry and ethanol distillery wastewater through biphasic high rate reactor. J. Environ. Sci. Health Part A 2018, 53, 676–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Wang, Y.; Sun, J.; Yan, T.; Li, J.; Zhao, T.; Yin, X.; Sun, C. Enhancement of biological treatment of wastewater by magnetic field. Bioresour. Technol. 2010, 101, 8535–8540. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, F.; Ou-Yang, F. Ethanol fermentation in a magnetically fluized bed reactor with immobilized Saccharomyces cerevisiae in magnetic particles. Bioresour. Technol. 2009, 100, 878–882. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Zhang, Y.; Ni, B.J. Zero valent iron significantly enhances methane production from waste activated sludge by improving biochemical methane potential rather than hydrolysis rate. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Karri, S.; Sierra-Alvarez, R.; Field, J.A. Zero valent iron as an electron-donor for methanogenesis and sulfate reduction in anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 810–819. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, S.; Ding, A.; Sun, G.; Yang, M. Performance of a zero valent iron-based anaerobic system in swine wastewater treatment. J. Hazard. Mater. 2015, 286, 1–6. [Google Scholar] [CrossRef]
- Lee, H.; Shoda, M. Stimulation of anaerobic digestion of thickened sewage sludge by iron-rich sludge produced by the Fenton method. J. Biosci. Bioeng. 2008, 106, 107–110. [Google Scholar] [CrossRef]
- Noubactep, C. A critical review on the process of contaminant removal in Fe0-H2O systems. Environ. Technol. 2008, 29, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysocka, I.; Krzemieniewski, M. The effects of total phosphorus and orthophosphates removal with the method of metals solubilisation on steel, aluminum, and mixed media. Pol. J. Nat. Sci. 2007, 22, 307–316. [Google Scholar] [CrossRef]
- Ruzhitskaya, O. Efficient phosphate removal by biological corrosion method. E3S Web Conf. 2020, 180, 04009. [Google Scholar] [CrossRef]
- Wysocka, I. A Comparative Study of Metals Solubilization and Electrocoagulation Methods’ Effectiveness in Orthophosphate Removal from Synthetic Wastewater. Pol. J. Environ. Stud. 2013, 22, 945–949. [Google Scholar]
- Tomei, M.C.; Stazi, V.; Daneshgar, S.; Capodaglio, A.G. Holistic Approach to Phosphorus Recovery from Urban Wastewater: Enhanced Biological Removal Combined with Precipitation. Sustainability 2020, 12, 575. [Google Scholar] [CrossRef] [Green Version]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Simulations and Laboratory Tests for Assessing Phosphorus Recovery Efficiency from Sewage Sludge. Resources 2018, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.G.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Berg, U.; Donnert, D.; Weidler, P.G.; Kaschka, E.; Knoll, G.; Nüesch, R. Phosphorus removal and recovery from wastewater by tobermoriteseeded crystallisation of calcium phosphate. Water Sci. Technol. 2006, 53, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallas, J.F.; Mackowiak, C.L.; Wilkie, A.C.; Harris, W.G. Struvite Phosphorus Recovery from Aerobically Digested Municipal Wastewater. Sustainability 2019, 11, 376. [Google Scholar] [CrossRef] [Green Version]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable Sewage Sludge Management: From Current Practices to Emerging Nutrient Recovery Technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef] [Green Version]
- Roeleveld, P.; Loeffen, P.; Temmink, H.; Klapwijk, B. Dutch analysis for Precovery from municipal wastewater. Water Sci. Technol. 2004, 49, 191–199. [Google Scholar] [CrossRef] [PubMed]










| Indicator mg/dm3 | Stage/Organic Load Rate | ||
|---|---|---|---|
| Stage 1—4.0 kg COD/m3·d | Stage 2—6.0 kg COD/m3·d | Stage 3—8.0 kg COD/m3·d | |
| COD | 3400 ± 201 | 4600 ± 190 | 6800 ± 270 |
| BOD5 | 1930 ± 170 | 2890 ± 154 | 3850 ± 206 |
| Total nitrogen | 130± 20 | 195 ± 22 | 260 ± 27 |
| COD/N | 26 ± 2 | 25 ± 2.2 | 26 ± 1.8 |
| Ammonia nitrogen | 20.4 ± 5.7 | 30.6 ± 8.3 | 40.8 ± 12 |
| Total phosphorus | 29.3 ± 4.9 | 43.9 ± 9.7 | 58.6 ± 9.1 |
| Orthophosphates | 4.2 ± 0.8 | 6.3 ± 1.6 | 8.4 ± 2.3 |
| Total suspended solids | 18.1 ± 1.9 | 27.2 ± 3.3 | 36.2 ± 5.2 |
| Stage | OLR [kg COD/m3·d] | Fraction of Raw Effluent [%] | Fraction of Tap Water [%] |
|---|---|---|---|
| Adaptation | 1.0 | 12.5 | 87.5 |
| 1 | 4.0 | 50 | 50 |
| 2 | 6.0 | 75 | 25 |
| 3 | 8.0 | 100 | 0 |
| Parameter | Unit | Average |
|---|---|---|
| pH | 7.44 ± 0.37 | |
| Water content | % | 97.9 ± 0.82 |
| Dry matter | % | 2.1 ± 0.82 |
| Volatile matter | % DM | 62.7 ± 3.01 |
| Ash | % DM | 47.3 ± 2.73 |
| Capillary suction time | s | 503 ± 28.29 |
| Property | Unit | Value |
|---|---|---|
| Density | [kg/m3] | 1230 ± 20 |
| Elastic modulus | [MPa] | 2600 ± 30 |
| Tensile strength | [MPa] | 21 ± 2 |
| Elongation at break | [%] | 300 ± 12 |
| Shore hardness A | [°Sh] | 80 ± 4 |
| Property | Unit | Value |
|---|---|---|
| Density | [kg/m3] | 874 ± 2 |
| Porosity | [%] | 40 ± 1 |
| Tensile strength | [MPa] | 14 ± 2 |
| Hardness | [°Sh] | 25 ± 3 |
| Parameter | Unit | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|---|
| Technological Parameters | ||||
| OLR | kg COD/m3·d | 4.0 | 6.0 | 8.0 |
| HRT | h | 24 | 24 | 24 |
| Operation time | d | 20 | 20 | 20 |
| Temperature | °C | 38 ± 1 | 38 ± 1 | 38 ± 1 |
| Results | ||||
| COD removal | % | 74.1 ± 7.0 | 74.9 ± 4.0 | 68.9 ± 4.0 |
| TP removal | % | 81.2 ± 8.2 | 69.8 ± 5.4 | 64.4 ± 2.4 |
| TN removal | % | 17.3 ± 4.0 | 11.4 ± 2.5 | 8.7 ± 3.0 |
| Biogas | dm3/kg CODremoved | 356 ± 25 | 427 ± 14 | 364 ± 11 |
| CH4 in biogas | % | 69.6 ± 1.4 | 72.0 ± 1.9 | 61.9 ± 3.1 |
| Daily biogas production | dm3/d | 638 ± 98 | 1134 ± 127 | 1206 ± 96 |
| Daily CH4 production | dm3CH4/d | 443 ± 64 | 817 ± 99 | 750 ± 91 |
| pH | 7.13 ± 0.16 | 7.05 ± 0.22 | 6.75 ± 0.18 | |
| FOS/TAC ratio | 0.31 ± 0.1 | 0.37 ± 0.1 | 0.44 ± 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Zieliński, M. Technological Effectiveness of Sugar-Industry Effluent Methane Fermentation in a Fluidized Active Filling Reactor (FAF-R). Energies 2020, 13, 6626. https://0-doi-org.brum.beds.ac.uk/10.3390/en13246626
Dębowski M, Zieliński M. Technological Effectiveness of Sugar-Industry Effluent Methane Fermentation in a Fluidized Active Filling Reactor (FAF-R). Energies. 2020; 13(24):6626. https://0-doi-org.brum.beds.ac.uk/10.3390/en13246626
Chicago/Turabian StyleDębowski, Marcin, and Marcin Zieliński. 2020. "Technological Effectiveness of Sugar-Industry Effluent Methane Fermentation in a Fluidized Active Filling Reactor (FAF-R)" Energies 13, no. 24: 6626. https://0-doi-org.brum.beds.ac.uk/10.3390/en13246626