Organic Carbon Is Ineffective in Enhancing the Growth of Dunaliella
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalga Strain and Cultivation Method
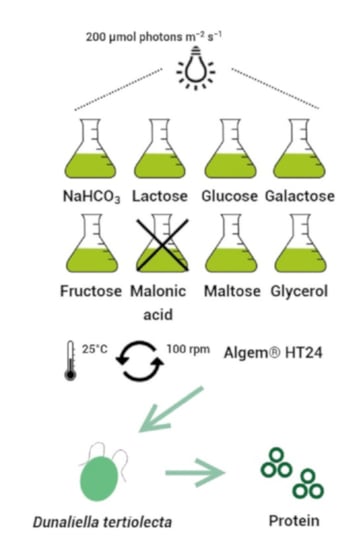
2.2. Preliminary Experiment
2.3. Mixotrophic Experiment
2.4. Sample Analysis
2.4.1. Cell Growth
2.4.2. Ash-Free Dry Weight
2.4.3. Nutrient Analysis
2.4.4. Amino Acids
2.4.5. Organic Carbon Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Experiments
3.2. Mixotrophic Experiment
3.2.1. Biomass Growth
3.2.2. Nutrient Recovery
3.2.3. Essential Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, Y.; Muys, M.; Van de Waal, D.B.; D’Adamo, S.; Vermeir, P.; Fernandes, T.V.; Vlaeminck, S.E. Enhancement of co-production of nutritional protein and carotenoids in Dunaliella salina using a two-phase cultivation assisted by nitrogen level and light intensity. Bioresour. Technol. 2019, 287, 121398. [Google Scholar] [CrossRef]
- Chen, H.H.; Xue, L.L.; Liang, M.H.; Jiang, J.G. Sodium azide intervention, salinity stress and two-step cultivation of Dunaliella tertiolecta for lipid accumulation. Enzym. Microb. Technol. 2019, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Vlaeminck, S.E. Dunaliella microalgae for nutritional protein: An undervalued asset. Trends Biotechnol. 2020, 38, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Avron, M.; Ben-Amotz, A. Production of Glycerol, Carotenes and Algae Meal. U.S. Patent 4199895A, 29 April 1980. [Google Scholar]
- Mordhay Avron, A.B.-A. Production of Glycerol from Algae. U.S. Patent 4115949A, 26 September 1978. [Google Scholar]
- Xu, Y.; Harvey, P.J. Phytoene and phytofluene overproduction by Dunaliella salina using the mitosis inhibitor chlorpropham. Algal Res. 2020, 52, 102126. [Google Scholar] [CrossRef]
- Sui, Y.; Harvey, P.J. Effect of Light Intensity and Wavelength on Biomass Growth and Protein and Amino Acid Composition of Dunaliella salina. Foods 2021, 10, 1018. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Research, M. Global Dunaliella Salina Market Research Report, Segment by Major Players, Types, Applications and Regions, 2016–2026; Maia Research: Bay Shore, NY, USA, 2021; p. 165. [Google Scholar]
- Díaz, J.P.; Inostroza, C.; Acién, F.G. Scale-up of a Fibonacci-Type Photobioreactor for the Production of Dunaliella salina. Appl. Biochem. Biotechnol. 2021, 193, 188–204. [Google Scholar] [CrossRef]
- Guldhe, A.; Kumari, S.; Ramanna, L.; Ramsundar, P.; Singh, P.; Rawat, I.; Bux, F. Prospects, recent advancements and challenges of different wastewater streams for microalgal cultivation. J. Environ. Manag. 2017, 203, 299–315. [Google Scholar] [CrossRef]
- Wu, K.-c.; Ho, K.-c.; Tang, C.-c.; Yau, Y.-h. The potential of foodwaste leachate as a phycoremediation substrate for microalgal CO2 fixation and biodiesel production. Environ. Sci. Pollut. Res. 2021, 28, 40724–40734. [Google Scholar] [CrossRef]
- Zaslavskaia, L.; Lippmeier, J.; Shih, C.; Ehrhardt, D.; Grossman, A.; Apt, K. Trophic conversion of an obligate photoautotrophic organism through metabolic engineering. Science 2001, 292, 2073–2075. [Google Scholar] [CrossRef]
- Kim, W.; Park, J.M.; Gim, G.H.; Jeong, S.-H.; Kang, C.M.; Kim, D.-J.; Kim, S.W. Optimization of culture conditions and comparison of biomass productivity of three green algae. Bioprocess Biosyst. Eng. 2012, 35, 19–27. [Google Scholar] [CrossRef]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int. J. Hydrog. Energy 2017, 42, 8505–8517. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Biomass and oil content of microalgae under mixotrophic conditions. Environ. Eng. Res. 2015, 20, 25–32. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- De Souza Celente, G.; Colares, G.S.; Machado, Ê.L.; Lobo, E.A. Algae turf scrubber and vertical constructed wetlands combined system for decentralized secondary wastewater treatment. Environ. Sci. Pollut. Res. 2019, 26, 9931–9937. [Google Scholar] [CrossRef]
- De Souza Celente, G.; Colares, G.S.; Da Silva Araújo, P.; Machado, Ê.L.; Lobo, E.A. Acute ecotoxicity and genotoxicity assessment of two wastewater treatment units. Environ. Sci. Pollut. Res. 2020, 27, 10520–10527. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Jang, H.M.; Kan, E. Microalgal Biomass and Lipid Production on Dairy Effluent Using a Novel Microalga, Chlorella sp. Isolated from Dairy Wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Venkata Mohan, S.; Nikhil, G.N.; Chiranjeevi, P.; Nagendranatha Reddy, C.; Rohit, M.V.; Kumar, A.N.; Sarkar, O. Waste biorefinery models towards sustainable circular bioeconomy: Critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [Google Scholar] [CrossRef]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzym. Microb. Technol. 2004, 34, 461–465. [Google Scholar] [CrossRef]
- Gim, G.H.; Ryu, J.; Kim, M.J.; Kim, P.I.; Kim, S.W. Effects of carbon source and light intensity on the growth and total lipid production of three microalgae under different culture conditions. J. Ind. Microbiol. Biotechnol. 2016, 43, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepanov, S.S.; Zolotareva, E.K. Methanol-induced stimulation of growth, intracellular amino acids, and protein content in Chlamydomonas reinhardtii. J. Appl. Phycol. 2015, 27, 1509–1516. [Google Scholar] [CrossRef]
- Rodríguez-Zavala, J.; Ortiz-Cruz, M.; Mendoza-Hernández, G.; Moreno-Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, F.; Ma, H.; Fan, Z.; Zhao, Z. The role of nitrobenzene on the yield of trihalomethane formation potential in aqueous solutions with Microcystis aeruginosa. Water Res. 2011, 45, 6489–6495. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodaei, S.; Abbasiliasi, S.; Shun, T.; Masoumi, H.F.; Mohamed, M.; Movahedi, A.; Rahim, R.; Ariff, A. Enhancement of protein production by microalgae Dunaliella salina under mixotrophic conditions using response surface methodology. RSC Adv. 2015, 5, 38141–38151. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Culture medium optimization for enhanced beta-carotene and biomass production by Dunaliella salina in mixotrophic culture. Biocatal. Agric. Biotechnol. 2016, 7, 217–223. [Google Scholar] [CrossRef]
- Chavoshi, Z.Z.; Shariati, M. Lipid production in Dunaliella salina under autotrophic, heterotrophic, and mixotrophic conditions. Biologia 2019, 74, 1579–1590. [Google Scholar] [CrossRef]
- Gonabadi, E.; Samadlouie, H.R.; Zenoozian, M.S. Optimization of culture conditions for enhanced Dunaliella salina productions in mixotrophic culture. Prep. Biochem. Biotechnol. 2022, 52, 154–162. [Google Scholar] [CrossRef]
- Wan, M.X.; Liu, P.; Xia, J.L.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.Y.; Qiu, G.Z. The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef]
- Bredda, E.H.; Da Silva, A.F.; Silva, M.B.; Da Ros, P.C.M. Mixture design as a potential tool in modeling the effect of light wavelength on Dunaliella salina cultivation: An alternative solution to increase microalgae lipid productivity for biodiesel production. Prep. Biochem. Biotechnol. 2020, 50, 379–389. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Gomaa, M. Pretreated fucoidan and alginate from a brown seaweed as a substantial carbon source for promoting biomass, lipid, biochemical constituents and biodiesel quality of Dunaliella salina. Renew. Energy 2020, 157, 246–255. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.M.; Liu, S.F.; Xu, R.L.; Mou, J.H.; Qin, Z.H.; Zhou, Z.G.; Li, H.Y.; Lin, C.S.K.; Sun, Z. Synergistic bioconversion of lipids and carotenoids from food waste by Dunaliella salina with fulvic acid via a two-stage cultivation strategy. Energy Convers. Manag. 2021, 234, 11. [Google Scholar] [CrossRef]
- Gastelum-Franco, J.J.; Esparza-Leal, H.M.; Garcia-Ulloa, M.; Lopez-Alvarez, E.S.; Muy-Rangel, M.D.; Perez-Rubio, V.; Ulloa-Mercado, R.G.; Montiel-Montoya, J. Preliminary evaluation of the green microalga Dunaliella salina as a potential feedstock for biodiesel: Effect of molasses on growth and lipid profile. Lat. Am. J. Aquat. Res. 2021, 49, 763–772. [Google Scholar] [CrossRef]
- Sohrabi, D.; Jazini, M.H.; Shariati, M. Mixotrophic Cultivation of Dunaliella salina on Crude Glycerol Obtained from Calcinated Fatty Acid Production Process. Russ. J. Mar. Biol. 2019, 45, 470–480. [Google Scholar] [CrossRef]
- Capa-Robles, W.; Garcia-Mendoza, E.; Paniagua-Michel, J.D. Enhanced beta-carotene and Biomass Production by Induced Mixotrophy in Dunaliella salina across a Combined Strategy of Glycerol, Salinity, and Light. Metabolites 2021, 11, 866. [Google Scholar] [CrossRef]
- Xie, Z.Z.; Lin, W.T.; Luo, J.F. Promotion of microalgal growth by co-culturing with Cellvibrio pealriver using xylan as feedstock. Bioresour. Technol. 2016, 200, 1050–1054. [Google Scholar] [CrossRef]
- Vidya, D.; Nayana, K.; Sreelakshmi, M.; Keerthi, K.V.; Mohan, K.S.; Sudhakar, M.P.; Arunkumar, K. A sustainable cultivation of microalgae using dairy and fish wastes for enhanced biomass and bio-product production. Biomass Convers. Biorefinery 2021, 1–15. [Google Scholar] [CrossRef]
- Kharati-Koupaei, M.; Moradshahi, A. Effects of Sodium Nitrate and Mixotrophic Culture on Biomass and Lipid Production in Hypersaline Microalgae Dunaliella Viridis Teod. Braz. Arch. Biol. Technol. 2016, 59, 9. [Google Scholar] [CrossRef] [Green Version]
- Chavoshi, Z.Z.; Shariati, M. Lipid production in Dunaliella bardawil under autotrophic, heterotrophic and mixotrophic conditions. Braz. J. Oceanogr. 2019, 67, 8. [Google Scholar] [CrossRef]
- Zanette, C.M.; Mariano, A.B.; Yukawa, Y.S.; Mendes, I.; Spier, M.R. Microalgae mixotrophic cultivation for -galactosidase production. J. Appl. Phycol. 2019, 31, 1597–1606. [Google Scholar] [CrossRef]
- Liang, M.H.; Xue, L.L.; Jiang, J.G. Two-Stage Cultivation of Dunaliella tertiolecta with Glycerol and Triethylamine for Lipid Accumulation: A Viable Way to Alleviate the Inhibitory Effect of Triethylamine on Biomass. Appl. Environ. Microbiol. 2019, 85, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzovenis, I.; Triantaphyllidis, G.; Naihong, X.; Chatzinikolaou, E.; Papadopoulou, K.; Xouri, G.; Tafas, T. Cryopreservation of marine microalgae and potential toxicity of cryoprotectants to the primary steps of the aquacultural food chain. Aquaculture 2004, 230, 457–473. [Google Scholar] [CrossRef]
- Rao, S.; Cultivation, D. Growth media, division rates and applications of Dunaliella species. In The Alga Dunaliella: Biodiversity, Physiology, Genomics and Biotechnology; Taylor & Francis: Oxfordshire, UK, 2009; pp. 44–89. [Google Scholar]
- Hadi, M.; Shariati, M.; Afsharzadeh, S. Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran. Biotechnol. Bioprocess Eng. 2008, 13, 540. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Borowitzka, L.J. Micro-Algal Biotechnology; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Sui, Y.; Mazzucchi, L.; Acharya, P.; Xu, Y.; Morgan, G.; Harvey, P.J. A Comparison of β-Carotene, Phytoene and Amino Acids Production in Dunaliella salina DF 15 (CCAP 19/41) and Dunaliella salina CCAP 19/30 Using Different Light Wavelengths. Foods 2021, 10, 2824. [Google Scholar] [CrossRef]
- Phenomenex. Phenomenex EZ: Faast Amino Acid Analysis; Phenomenex: Torrance, CA, USA, 2013. [Google Scholar]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of Australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhong, C.; Wu, X.-Y.; Wei, Y.-Q.; Bo, T.; Han, P.-P.; Jia, S.-R. Metabolomic profiling coupled with metabolic network reveals differences in Gluconacetobacter xylinus from static and agitated cultures. Biochem. Eng. J. 2015, 101, 85–98. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.; Ryan, P.D. Palaeontological statistics software package for education and data analysis. Palaeontol. Electron 2001, 4, 1–9. [Google Scholar]
- Liang, Y.; Abbott, D.; Howard, N.; Lim, K.; Ward, R.; Elgendi, M. How effective is pulse arrival time for evaluating blood pressure? Challenges and recommendations from a study using the MIMIC database. J. Clin. Med. 2019, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Hard, B.C.; Gilmour, D.J. The uptake of organic compounds by Dunaliella parva CCAP 19/9. Eur. J. Phycol. 1996, 31, 217–224. [Google Scholar] [CrossRef]
- Raven, J.A.; Beardall, J. Carbohydrate metabolism and respiration in algae. In Photosynthesis in Algae; Springer: Berlin/Heidelberg, Germany, 2003; pp. 205–224. [Google Scholar]
- Markou, G.; Angelidaki, I.; Georgakakis, D. Microalgal carbohydrates: An overview of the factors influencing carbohydrates production, and of main bioconversion technologies for production of biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Farrelly, D.J.; Brennan, L.; Everard, C.D.; McDonnell, K.P. Carbon dioxide utilisation of Dunaliella tertiolecta for carbon bio-mitigation in a semicontinuous photobioreactor. Appl. Microbiol. Biotechnol. 2014, 98, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q.; Chen, F. Growing phototrophic cells without light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mujtaba, G.; Lee, K. Influence of organic carbon sources on growth and lipid content of marine green alga Dunaliella tertiolecta. J. Mar. Biosci. Biotechnol. 2014, 6, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Keerthi, S.; Koduru, U.D.; Nittala, S.S.; Parine, N.R. The heterotrophic eubacterial and archaeal co-inhabitants of the halophilic Dunaliella salina in solar salterns fed by Bay of Bengal along south eastern coast of India. Saudi J. Biol. Sci. 2018, 25, 1411–1419. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.-H.; Oh, H.-M.; Kim, H.-S. Enhancing microalgal biomass productivity by engineering a microalgal–bacterial community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol production from microalgae polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef]
- Kim, G.-Y.; Heo, J.; Kim, H.-S.; Han, J.-I. Bicarbonate-based cultivation of Dunaliella salina for enhancing carbon utilization efficiency. Bioresour. Technol. 2017, 237, 72–77. [Google Scholar] [CrossRef]
- Hu, S.; Wang, Y.; Wang, Y.; Zhao, Y.; Zhang, X.; Zhang, Y.; Jiang, M.; Tang, X. Effects of elevated pCO2 on physiological performance of marine microalgae Dunaliella salina (Chlorophyta, Chlorophyceae). J. Oceanol. Limnol. 2018, 36, 317–328. [Google Scholar] [CrossRef]
- Booth, W.A.; Beardall, J. Effects of salinity on inorganic carbon utilization and carbonic anhydrase activity in the halotolerant alga Dunaliella salina (Chlorophyta). Phycologia 1991, 30, 220–225. [Google Scholar] [CrossRef]
- Oren, A. A hundred years of Dunaliella research: 1905–2005. Saline Syst. 2005, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Liska, A.J.; Shevchenko, A.; Pick, U.; Katz, A. Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol. 2004, 136, 2806–2817. [Google Scholar] [CrossRef] [Green Version]
- Aizawa, K.; Miyachi, S. Carbonic anhydrase located on cell surface increases the affinity for inorganic carbon in photosynthesis of Dunaliella tertiolecta. FEBS letters 1984, 173, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Andreeva, A.; Budenkova, E.; Babich, O.; Sukhikh, S.; Dolganyuk, V.; Michaud, P.; Ivanova, S. Influence of Carbohydrate Additives on the Growth Rate of Microalgae Biomass with an Increased Carbohydrate Content. Mar. Drugs 2021, 19, 381. [Google Scholar] [CrossRef]
- Soltani Nezhad, F.; Mansouri, H. Effects of polyploidy on response of Dunaliella salina to salinity. J. Mar. Biol. Assoc. U. K. 2019, 99, 1041–1047. [Google Scholar] [CrossRef]
- Qie, F.; Zhu, J.; Rong, J.; Zong, B. Biological removal of nitrogen oxides by microalgae, a promising strategy from nitrogen oxides to protein production. Bioresour. Technol. 2019, 292, 122037. [Google Scholar] [CrossRef]
- Fan, J.; Cui, Y.; Wan, M.; Wang, W.; Li, Y. Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosaunder three nutrition stressors. Biotechnol. Biofuels 2014, 7, 17. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, V.; Solimeno, A.; Chindris, A.; Marazzi, F.; García, J. Growth of Tetraselmis suecica and Dunaliella tertiolecta in Aquaculture Wastewater: Numerical Simulation with the BIO_ALGAE Model. Water Air Soil Pollut. 2019, 230, 60. [Google Scholar] [CrossRef]
- Wu, K.-c.; Yau, Y.-h.; Sze, E.T.-P. Application of anaerobic bacterial ammonification pretreatment to microalgal food waste leachate cultivation and biofuel production. Mar. Pollut. Bull. 2020, 153, 111007. [Google Scholar] [CrossRef]
- Lv, J.; Guo, J.; Feng, J.; Liu, Q.; Xie, S. Effect of sulfate ions on growth and pollutants removal of self-flocculating microalga Chlorococcum sp. GD in synthetic municipal wastewater. Bioresour. Technol. 2017, 234, 289–296. [Google Scholar] [CrossRef]
- Zhou, H.; Sheng, Y.; Zhao, X.; Gross, M.; Wen, Z. Treatment of acidic sulfate-containing wastewater using revolving algae biofilm reactors: Sulfur removal performance and microbial community characterization. Bioresour. Technol. 2018, 264, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, S. Regulation of Sulfate Assimilation in Arabidopsis and Beyond. Ann. Bot. 2006, 97, 479–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Martinez, A.; Garcia, N.M.; Romero, I.; Seco, A.; Ferrer, J. Microalgae cultivation in wastewater: Nutrient removal from anaerobic membrane bioreactor effluent. Bioresour. Technol. 2012, 126, 247–253. [Google Scholar] [CrossRef]
- Huertas, E.; Montero, O.; Lubián, L.M. Effects of dissolved inorganic carbon availability on growth, nutrient uptake and chlorophyll fluorescence of two species of marine microalgae. Aquac. Eng. 2000, 22, 181–197. [Google Scholar] [CrossRef]
- Su, Y. Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Mageswari, A.; Subramanian, P.; Suganthi, C.; Chaitanyakumar, A.; Aswini, V.; Gothandam, K.M. Bicarbonate supplementation enhances growth and biochemical composition of Dunaliella salina V-101 by reducing oxidative stress induced during macronutrient deficit conditions. Sci. Rep. 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, R.; Kumar, V.A.; Kumar, D.; Ramesh, N.; Babu, S.; Gothandam, K.M. Effect of Dissolved Inorganic Carbon on β-Carotene and Fatty Acid Production in Dunaliella sp. Appl. Biochem. Biotechnol. 2015, 175, 2895–2906. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, J.; Xue, S.; Chi, Z. β-Carotene production from Dunaliella salina cultivated with bicarbonate as carbon source. J. Microbiol. Biotechnol. 2020, 30, 868–877. [Google Scholar] [CrossRef]





| Quality | Range |
|---|---|
| High | EAAI > 0.95 |
| Good | 0.86 < EAAI ≤ 0.95 |
| Useful | 0.75 < EAAI ≤ 0.86 |
| Inadequate | EAAI ≤ 0.75 |
| Strength of Correlation | Range of Absolute Correlation Coefficient (r) |
|---|---|
| Very strong | 0.8–1.0 |
| Strong | 0.6–0.79 |
| Moderate | 0.4–0.59 |
| Weak | 0.2–0.39 |
| Very weak | 0–0.19 |
| Treatment | Cell Density Cell mL−1 | AFDW g L−1 |
|---|---|---|
| NaHCO3 | 4.64 × 106 ± 1.81 × 105 a | 1.43 ± 0.02 a |
| Lactose | 3.85 × 106 ± 4.89 × 105 ab | 1.29 ± 0.05 b |
| Glucose | 3.12 × 106 ± 4.61 × 105 b | 1.10 ± 0.02 c |
| Galactose | 3.65 × 106 ± 4.98 × 105 ab | 1.17 ± 0.03 bc |
| Fructose | 3.40 × 106 ± 1.94 × 105 b | 1.12 ± 0.05 c |
| Glycerol | 3.25 × 106 ± 2.10 × 105 b | 1.12 ± 0.03 c |
| Maltose | 3.64 × 106 ± 4.76 × 105 ab | 1.11 ± 0.05 c |
| Treatment | Recovery (%) | Organic Carbon Relative Content | ||
|---|---|---|---|---|
| PO4-P | NO3-N | SO4-S | ||
| NaHCO3 | 100 | 91.76 ± 2.71 ab | 4.46 ± 6.30 | - |
| Lactose | 100 | 90.03 ± 8.47 ab | ND | 0.97 ± 0.07 |
| Glucose | 100 | 90.03 ± 8.47 ab | 6.67 ± 5.46 | 0.97 ± 0.04 |
| Galactose | 100 | 74.52 ± 3.58 ab | 9.69 ± 13.71 | 0.99 ± 0.23 |
| Fructose | 100 | 96.93 ± 4.34 ab | 5.92 ± 4.57 | 0.98 ± 0.23 |
| Glycerol | 100 | 99.81 ± 0.27 a | 11.91 ± 12.44 | 0.96 ± 0.07 |
| Maltose | 100 | 61.11 ± 25.84 b | 1.47 ± 2.07 | 0.95 ± 0.09 |
| Treatment | EAA Concentration (mg g−1) | EAA (%AA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Valine | Leucine | Isoleucine | Threonine | Methionine | Phenylalanine + Tyrosine | Lysine | Histidine | ||
| NaHCO3 | 56 | 101 | 54 | 59 | 18 | 103 | 74 | 47 | 51 |
| Lactose | 57 | 98 | 57 | 59 | 18 | 96 | 55 | 28 | 47 |
| Glucose | 58 | 97 | 60 | 61 | 20 | 101 | 56 | 33 | 49 |
| Galactose | 55 | 97 | 58 | 61 | 17 | 96 | 65 | 30 | 48 |
| Fructose | 62 | 98 | 61 | 55 | 20 | 95 | 54 | 30 | 47 |
| Glycerol | 54 | 87 | 58 | 55 | 20 | 115 | 62 | 41 | 49 |
| Maltose | 59 | 97 | 53 | 59 | 19 | 93 | 66 | 34 | 48 |
| FAO/WHO reference | 39 | 59 | 30 | 23 | 16 | 38 | 45 | 15 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celente, G.d.S.; Rizzetti, T.M.; Schneider, R.d.C.d.S.; Harvey, P.J.; Sui, Y. Organic Carbon Is Ineffective in Enhancing the Growth of Dunaliella. Fermentation 2022, 8, 261. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8060261
Celente GdS, Rizzetti TM, Schneider RdCdS, Harvey PJ, Sui Y. Organic Carbon Is Ineffective in Enhancing the Growth of Dunaliella. Fermentation. 2022; 8(6):261. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8060261
Chicago/Turabian StyleCelente, Gleison de Souza, Tiele Medianeira Rizzetti, Rosana de Cassia de Souza Schneider, Patricia J. Harvey, and Yixing Sui. 2022. "Organic Carbon Is Ineffective in Enhancing the Growth of Dunaliella" Fermentation 8, no. 6: 261. https://0-doi-org.brum.beds.ac.uk/10.3390/fermentation8060261