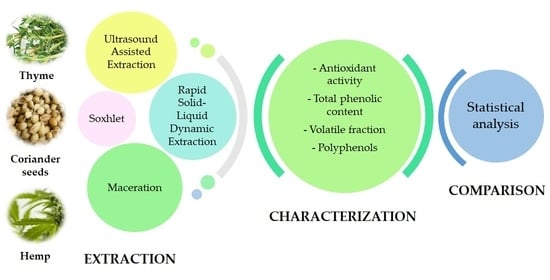
Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Extractions
2.3.1. RSLDE Extraction
2.3.2. Soxhlet Extraction
2.3.3. Maceration
2.3.4. UAE Extraction
2.4. SPME/GC–MS Characterization of Extracts Volatile Fraction
2.5. HPLC-UV Characterization of the Phenolic Fraction
2.6. Total Phenolic Content (TPC) and Antioxidant Capacity (AOC)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yields
3.2. Chemical Composition of Extracts Volatile Fraction
3.3. Polyphenolic Composition
3.4. Total Phenolic Content and Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajkarimi, M.; Ibrahim, S.A.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef] [PubMed]
- Briskin, D.P. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000, 124, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaino, A.; Cimino, F.; Zimbalatti, V.; Venuti, V.; Sulfaro, V.; De Pasquale, A.; Saija, A. Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chem. 2005, 89, 549–554. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Smith, R.M. Before the injection—Modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present Panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Prasad Devkota, H.; Erdogan Orhan, I.; Kumar Patra, J.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Naviglio, D. Naviglio’s principle and presentation of an innovative solid–liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Gallo, M.; Vitulano, M.; Andolfi, A.; Della Greca, M.; Conte, E.; Ciaravolo, M.; Naviglio, D. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A new rapid and greener method for extracting two steviol glycosides (Stevioside and Rebaudioside A) from stevia leaves. Plant Foods Hum. Nutr. 2017, 72, 141–148. [Google Scholar] [CrossRef]
- Ferrara, L.; Naviglio, D.; Gallo, M. Extraction of bioactive compounds of saffron (Crocus sativus L.) by ultrasound assisted extraction (UAE) and by rapid solid-liquid dynamic extraction (RSLDE). Eur. Sci. J. 2014, 10, 1–13. [Google Scholar]
- Cozzolino, I.; Vitulano, M.; Conte, E.; D’Onofrio, F.; Aletta, L.; Ferrara, L.; Andolfi, A.; Naviglio, D.; Gallo, M. Extraction and curcuminoids activity from the roots of Curcuma longa by RSLDE using the Naviglio extractor. Eur. Sci. J. (Special Edition) 2016, 12, 119–127. [Google Scholar]
- Gallo, M.; Formato, A.; Ciaravolo, M.; Langella, C.; Cataldo, R.; Naviglio, D. A water extraction process for lycopene from tomato waste using a pressurized method: An application of a numerical simulation. Eur. Food Res. Technol. 2019, 245, 1767–1775. [Google Scholar] [CrossRef]
- Lee, K.-G.; Shibamoto, T. Antioxidant properties of aroma compounds isolated from soybeans and mung beans. J. Agric. Food Chem. 2000, 48, 4290–4293. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Barber, X.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Bioaccessibility, changes in the antioxidant potential and colonic fermentation of date pits and apple bagasse flours obtained from co-products during simulated in vitro gastrointestinal digestion. Food Res. Int. 2015, 78, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Zekovic, Z.P. Analysis of thyme (Thymus vulgaris L.) extracts. Acta Period. Technol. Yugosl. 2000, 31, 617–622. [Google Scholar]
- Zhang, Z.-M.; Li, G.-K. A preliminary study of plant aroma profile characteristics by a combination sampling method coupled with GC–MS. Microchem. J. 2007, 86, 29–36. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.-N.; Liu, Z.-H.; Zhao, Y.-P.; Zhao, L.-L.; Xue, T.-K.; Lan, Q.-K. Phytochemical and Bioactive Profile of Coriandrum sativum L. Food Chem. 2019, 286, 260–267. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Guillen, M.; Manzanos, M. Study of the composition of the different parts of a Spanish Thymus vulgaris L. plant. Food Chem. 1998, 63, 373–383. [Google Scholar] [CrossRef]
- Cappelletto, P.; Brizzi, M.; Mongardini, F.; Barberi, B.; Sannibale, M.; Nenci, G.; Poli, M.; Corsi, G.; Grassi, G.; Pasini, P. Italy-grown hemp: Yield, composition and cannabinoid content. Ind. Crops Prod. 2001, 13, 101–113. [Google Scholar] [CrossRef]
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella sativa L. and Thymus vulgaris L., and its anti-proliferative effect on HeLa cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Karam, L.; Roustom, R.; Abiad, M.G.; El-Obeid, T.; Savvaidis, I.N. Combined effects of thymol, carvacrol and packaging on the shelf-life of marinated chicken. Int. J. Food Microbiol. 2019, 291, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Flores, P.I.G.; Valenzuela, R.B.; Ruiz, L.D.; López, C.M.; Cháirez, F.E. Antibacterial activity of five terpenoid compounds: Carvacrol, limonene, linalool, α-terpinene and thymol. Trop. Subtrop. Agroecosyst. 2019, 22, 443–450. [Google Scholar]
- Agatonovic-Kustrin, S.; Kustrin, E.; Morton, D.W. Essential oils and functional herbs for healthy aging. Neural Regen. Res. 2019, 14, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Tambe, Y.; Tsujiuchi, H.; Honda, G.; Ikeshiro, Y.; Tanaka, S. Gastric Cytoprotection of the Non-Steroidal Anti-Inflammatory Sesquiterpene, β-Caryophyllene. Planta Med. 1996, 62, 469–470. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L.(hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [Green Version]
- Stenerson, K.K.; Halpenny, M.R. Analysis of Terpenes in Cannabis Using Headspace Solid-Phase Microextraction and GC–MS. LCGC North Am. 2017, 35, 28. [Google Scholar]
- Jana, S.; Patra, K.; Sarkar, S.; Jana, J.; Mukherjee, G.; Bhattacharjee, S.; Mandal, D.P. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: An in vivo study in sarcoma-180 solid tumor model. Nutr. Cancer 2014, 66, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Viljoen, A. Geraniol—A review of a commercially important fragrance material. South Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Rossi, C.; Palmieri, S.; Maggio, F.; Chaves, C.; Sterzo, C.L.; Paparella, A.; de Medici, D.; Ricci, A.; Serio, A. Salmonella enterica Control in Stick Carrots through Incorporation of Coriander Seeds Essential Oil in Sustainable Washing Treatments. Front. Sustain. Food Syst. 2020, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gębarowska, E.; Pytlarz-Kozicka, M.; Nöfer, J.; Łyczko, J.; Adamski, M.; Szumny, A. The Effect of Trichoderma spp. on the Composition of Volatile Secondary Metabolites and Biometric Parameters of Coriander (Coriandrum sativum L.). J. Food Qual. 2019, 2019. Article ID 5687032, 7p. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of essential oils obtained from Abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonca, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; de Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Eucalyptus leaves using solvents of distinct polarity. Cluster analysis and extracts characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Vural, N.; Algan-Cavuldak, Ö.; Akay, M.A.; Anli, R.E. Determination of the various extraction solvent effects on polyphenolic profile and antioxidant activities of selected tea samples by chemometric approach. J. Food Meas. and Charact. 2020, 14, 1286–1305. [Google Scholar] [CrossRef]
- Armatu, A.; Colceru-Mihul, S.; Bubueanu, C.; Draghici, E.; Pirvu, L. Evaluation of antioxidant and free scavenging potential of some Lamiaceae species growing in Romania. Rom. Biotechnol. Lett. 2010, 15, 5274–5280. [Google Scholar]
- Chaouche, T.M.; Haddouchi, F.; Ksouri, R.; Medini, F.; El-Haci, I.A.; Boucherit, Z.; Sekkal, F.Z.; Atik-Bekara, F. Antioxidant Potential of Hydro-methanolic Extract of Prasium majus L: An in vitro Study. Pak. J. Biol. Sci. 2013, 16, 1318–1323. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.-A.; Rahaman, A.; Aadil, R.M.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maltese, F.; van der Kooy, F.; Verpoorte, R. Solvent Derived Artifacts in Natural Products Chemistry. Nat. Prod. Commun. 2009, 4, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Vaya, J.; Mahmood, S.; Goldblum, A.; Aviram, M.; Volkova, N.; Shaalan, A.; Musa, R.; Tamir, S. Inhibition of LDL oxidation by flavonoids in relation to their structure and calculated enthalpy. Phytochemistry 2003, 62, 89–99. [Google Scholar] [CrossRef]
- Bazylko, A.; Strzelecka, H. A HPTLC densitometric determination of luteolin in Thymus vulgaris and its extracts. Fitoterapia 2007, 78, 391–395. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. Essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Duenas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C. Phenolic profiles of in vivo and in vitro grown Coriandrum sativum L. Food Chem. 2012, 132, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Msaada, K.; Jemia, M.B.; Salem, N.; Bachrouch, O.; Sriti, J.; Tammar, S.; Bettaieb, I.; Jabri, I.; Kefi, S.; Limam, F. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arab. J. Chem. 2017, 10, CS3176–CS3183. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Sena, S.R.; Dantas, T.R.; Pereira, C.G. Extracts from Thymus vulgaris and Origanum vulgare L. obtained by different separation processes: Global yield and functional profile. Trends Phytochem. Res. 2018, 2, 13–20. [Google Scholar]
- Sriti, J.; Wannes, W.A.; Talou, T.; Jemia, M.B.; Kchouk, M.E.; Marzouk, B. Antioxidant properties and polyphenol contents of different parts of coriander (Coriandrum sativum L.) fruit. La Rivista Italiana Delle Sostanze Grasse 2012, 49, 253–262. [Google Scholar]
- Moccia, S.; Siano, F.; Russo, G.L.; Volpe, M.G.; La Cara, F.; Pacifico, S.; Piccolella, S.; Picariello, G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L., Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2020, 71, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Galati, E.M.; Monforte, M.T.; Lanuzza, F.; D’Angelo, V.; Circosta, C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016, 30, 1298–1307. [Google Scholar] [CrossRef]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene Thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; van Boekel, M.A.J.S. Activity and concentration of polyphenolic antioxidants in apple juice. 3. Stability during storage. J. Agric. Food Chem. 2005, 53, 1073–1080. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, L.; Moore, J.; Zhou, K.; Luther, M.; Yin, J.-J.; Yu, L. Effects of postharvest treatment and heat stress on availability of wheat antioxidants. J. Agric. Food Chem. 2006, 54, 5623–5629. [Google Scholar] [CrossRef]
- Dutra, R.; Leite, M.; Barbosa, N. Quantification of phenolic constituents and antioxidant activity of Pterodon Emarginatus vogel seeds. Int. J. Mol. Sci. 2008, 9, 606. [Google Scholar] [CrossRef] [Green Version]
- Antolovich, M.; Prenzler, P.; Robards, K.; Ryan, D. Sample preparation in the determination of phenolic compounds in fruits. Analyst 2000, 125, 989–1009. [Google Scholar] [CrossRef]




| N2h | N6h | S2h | S6h | UAE | M | |
|---|---|---|---|---|---|---|
| Thyme | 2.30 ± 0.06 d | 2.45 ± 0.09 c | 9.25 ± 0.02 a | 8.65 ± 0.05 b | 1.62 ± 0.02 e | 1.78 ± 0.04 e |
| Hemp | 6.00 ± 0.03 b | 5.81 ± 0.05 b | 5.70 ± 0.01 b | 10.00 ± 0.07 a | 0.71 ± 0.09 c | 0.95 ± 0.04 c |
| Coriander seeds | 0.57 ± 0.09 f | 0.73 ± 0.08 e | 1.18 ± 0.06 d | 1.63 ± 0.09 c | 2.36 ± 0.07 a | 2.17 ± 0.08 b |
| ID-Thyme | Terpenes Class | RI | N2h | N6h | S2h | S6h | UAE | M |
| α-pinene | bicyclic monoterpenes | 921 | 2.11 ± 0.73 | 2.34 ± 0.31 | 2.53 ± 0.19 | 2.26 ± 0.10 | 2.47 ± 0.35 | 1.57 ± 0.16 |
| sabinene | bicyclic monoterpenes | 967 | 2.49 ± 0.61 | 2.82 ± 0.27 | 2.36 ± 0.09 | 2.90 ± 0.22 | 3.04 ± 0.22 | 2.21 ± 0.25 |
| 1-octen-3-ol | acyclic monoterpenes | 980 | 0.63 ± 0.02 | 0.31 ± 0.01 | 0.30 ± 0.00 | 0.61 ± 0.04 | 0.82 ± 0.13 | 0.22 ± 0.00 |
| β-myrcene | acyclic monoterpenes | 999 | 0.52 ± 0.02 | 0.56 ± 0.08 | 0.82 ± 0.23 | 0.60 ± 0.01 | 0.56 ± 0.03 | 0.43 ± 0.00 |
| ɣ-3-carene | bicyclic monoterpenes | 1010 | 3.52 ± 0.38 | 4.20 ± 0.94 | 3.26 ± 0.96 | 4.16 ± 0.04 | 4.10 ± 0.19 | 3.15 ± 0.15 |
| o-cymene | monocyclic monoterpenes | 1019 | 0.18 ± 0.01 | 0.90 ± 0.08 | 1.24 ± 0.54 | 0.45 ± 0.08 | 1.31 ± 0.31 | 0.16 ± 0.01 |
| p-cymene | monocyclic monoterpenes | 1023 | 1.18 ± 0.04 | 1.33 ± 0.20 | 7.81 ± 0.26 | 1.38 ± 0.10 | 1.34 ± 0.03 | 1.01 ± 0.04 |
| ɣ-terpinene | monocyclic monoterpenes | 1054 | 5.45 ± 0.49 | 3.21 ± 0.54 | 5.48 ± 0.16 | 7.17 ± 0.80 | 6.81 ± 0.19 | 5.03 ± 0.22 |
| cis-sabinene hydrate | bicyclic monoterpenes | 1066 | 5.93 ± 0.76 | 4.88 ± 0.26 | 2.29 ± 0.53 | 5.06 ± 0.42 | 5.96 ± 0.05 | 4.14 ± 0.09 |
| cis-linalool oxide | monocyclic monoterpenes | 1079 | 1.14 ± 0.14 | 1.30 ± 0.27 | 5.68 ± 0.33 | 1.33 ± 0.06 | 1.20 ± 0.04 | 1.03 ± 0.01 |
| linalool | acyclic monoterpenes | 1096 | 48.19 ± 1.95 | 46.32 ± 1.23 | 16.19 ± 2.88 | 42.59 ± 1.37 | 47.66 ± 3.79 | 44.95 ± 1.81 |
| cis-p-menth-2-en-1-ol | monocyclic monoterpenes | 1123 | 2.03 ± 0.65 | 0.81 ± 0.02 | 14.36 ± 0.19 | 0.37 ± 0.05 | 0.68 ± 0.01 | 0.19 ± 0.01 |
| trans-limonene oxide | monocyclic monoterpenes | 1138 | 2.20 ± 0.54 | 0.89 ± 0.11 | 1.24 ± 1.68 | 0.15 ± 0.02 | 0.19 ± 0.07 | 0.40 ± 0.00 |
| β-pinene oxide | bicyclic monoterpenes | 1155 | 0.01 ± 0.00 | 1.06 ± 0.01 | 6.92 ± 0.86 | 0.36 ± 0.04 | 0.79 ± 0.02 | 0.30 ± 0.00 |
| trans-linalool oxide | monocyclic monoterpenes | 1174 | 5.09 ± 0.01 | 5.00 ± 0.76 | 6.36 ± 0.67 | 5.25 ± 0.25 | 6.75 ± 0.24 | 3.24 ± 0.27 |
| α-terpineol | monocyclic monoterpenes | 1190 | 3.07 ± 0.46 | 3.46 ± 0.47 | 1.44 ± 0.28 | 3.57 ± 0.12 | 4.28 ± 1.17 | 3.54 ± 0.11 |
| trans-piperitol | monocyclic monoterpenes | 1208 | 0.71 ± 0.14 | 0.44 ± 0.03 | 4.76 ± 0.65 | 0.19 ± 0.00 | 0.21 ± 0.03 | 0.19 ± 0.15 |
| 6,7-epoxigeranial | acyclic monoterpenes | 1232 | 0.76 ± 0.03 | 0.65 ± 0.01 | 0.85 ± 0.15 | 0.39 ± 0.11 | 0.26 ± 0.10 | 0.32 ± 0.01 |
| carvone | monocyclic monoterpenes | 1243 | 0.76 ± 0.01 | 0.3 ± 0.01 | 0.05 ± 0.01 | 0.66 ± 0.06 | 1.01 ± 0.42 | 0.45 ± 0.02 |
| linalyl acetate | acyclic monoterpenes | 1247 | 2.49 ± 0.20 | 1.71 ± 0.64 | 2.74 ± 0.70 | 3.35 ± 0.27 | 2.82 ± 0.69 | 2.56 ± 0.14 |
| carvacrol | monocyclic monoterpenes | 1296 | 9.18 ± 2.01 | 15.84 ± 1.09 | 6.52 ± 2.26 | 13.99 ± 2.68 | 5.49 ± 0.75 | 21.30 ± 0.72 |
| β-bisabolene | sesquiterpenes | 1508 | 1.29 ± 0.31 | 0.67 ± 0.01 | 2.00 ± 0.49 | 1.50 ± 0.23 | 1.21 ± 0.50 | 1.70 ± 0.08 |
| D-Hemp | Terpenes Class | RI | N2h | N6h | S2h | S6h | UAE | M |
| α-thuyene | bicyclic monoterpenes | 898 | 0.07 ± 0.00 | 0.06 ± 0.02 | 0.02 ± 0.0 | 0.02 ± 0.0 | - | 0.13 ± 0.01 |
| α-pinene | bicyclic monoterpenes | 915 | 0.09 ± 0.00 | 0.10 ± 0.04 | 0.40 ± 0.1 | 0.16 ± 0.1 | - | 0.22 ± 0.04 |
| β-pinene | bicyclic monoterpenes | 959 | 0.16 ± 0.01 | 0.15 ± 0.00 | 0.34 ± 0.0 | 0.14 ± 0.0 | 0.28 ± 0.03 | 0.20 ± 0.03 |
| β-myrcene | acyclic monoterpenes | 969 | 2.47 ± 0.29 | 0.78 ± 0.11 | 4.27 ± 0.04 | 3.03 ± 0.8 | - | 1.98 ± 0.14 |
| D-limonene | monocyclic monoterpenes | 1011 | 0.65 ± 0.34 | 0.26 ± 0.02 | 0.55 ± 0.01 | 0.36 ± 0.0 | 0.10 ± 0.03 | 0.61 ± 0.05 |
| eucaliptol | bicyclic monoterpenes | 1016 | 0.86 ± 0.41 | 0.46 ± 0.07 | 1.01 ± 0.0 | 0.74 ± 0.1 | 0.11 ± 0.02 | 2.14 ± 0.26 |
| β-ocymene | acyclic monoterpenes | 1027 | 0.95 ± 0.65 | 0.15 ± 0.04 | 2.27 ± 0.1 | 1.11 ± 0.3 | 2.25 ± 0.08 | 0.52 ± 0.01 |
| ɣ-terpinene | monocyclic monoterpenes | 1039 | 0.41 ± 0.15 | 0.89 ± 0.11 | 0.46 ± 0.00 | 0.25 ± 0.00 | 0.32 ± 0.05 | 1.66 ± 0.07 |
| terpinolene | monocyclic monoterpenes | 1067 | 3.75 ± 0.17 | 0.37 ± 0.04 | 3.79 ± 0.03 | 5.05 ± 0.9 | 0.03 ± 0.00 | 2.15 ± 0.72 |
| linalool | acyclic monoterpenes | 1079 | 4.93 ± 0.30 | 9.42 ± 1.67 | 2.69 ± 0.04 | 0.63 ± 0.0 | 0.26 ± 0.04 | 25.98 ± 1.73 |
| L-trans-pinocarveol | bicyclic monoterpenes | 1109 | 0.87 ± 0.36 | 0.75 ± 0.01 | 0.42 ± 0.1 | 0.60 ± 0.1 | 0.81 ± 0.15 | 0.60 ± 0.06 |
| cis-p-mentha-2,8-dien-1-ol | monocyclic monoterpenes | 1279 | 1.58 ± 0.39 | 1.23 ± 0.10 | 0.92 ± 0.2 | 1.35 ± 0.0 | 0.23 ± 0.04 | 1.20 ± 0.01 |
| geranyl acetate | acyclic monoterpenes | 1372 | 1.00 ± 0.16 | 0.72 ± 0.01 | 1.46 ± 0.59 | 1.53 ± 0.36 | 0.96 ± 0.63 | 1.50 ± 0.18 |
| caryophyllene | sesquiterpenes | 1390 | 51.85 ± 2.57 | 52.78 ± 2.61 | 52.44 ± 0.1 | 54.78 ± 0.1 | 40.00 ± 0.36 | 39.61 ± 3.31 |
| α-bergamotene | sesquiterpenes | 1400 | 5.86 ± 1.26 | 6.14 ± 0.51 | 4.83 ± 0.0 | 6.53 ± 0.2 | 4.79 ± 0.22 | 2.56 ± 0.52 |
| cis-β-farnesene | sesquiterpenes | 1416 | 3.82 ± 0.50 | 3.54 ± 0.42 | 2.70 ± 0.1 | 4.20 ± 0.3 | 4.03 ± 0.35 | 2.18 ± 0.18 |
| humulene | sesquiterpenes | 1423 | 13.60 ± 1.81 | 13.49 ± 0.14 | 12.11 ± 0.2 | 14.13 ± 0.4 | 12.25 ± 0.99 | 8.67 ± 0.05 |
| aromadendrene | sesquiterpenes | 1428 | 1.85 ± 0.18 | 2.86 ± 0.12 | 2.35 ± 0.0 | 2.09 ± 0.1 | 3.16 ± 0.19 | 2.55 ± 0.01 |
| β-selinene | sesquiterpenes | 1459 | 1.96 ± 0.79 | 2.81 ± 0.17 | 2.39 ± 0.1 | 0.96 ± 0.6 | 0.53 ± 0.10 | 3.37 ± 0.39 |
| α-selinene | sesquiterpenes | 1466 | 1.14 ± 0.86 | 1.91 ± 0.15 | 1.63 ± 0.0 | 1.12 ± 0.1 | 3.26 ± 0.59 | 2.30 ± 0.25 |
| ɣ-cadinene | sesquiterpenes | 1408 | 0.32 ± 0.11 | 0.20 ± 0.00 | 0.40 ± 0.0 | 0.41 ± 0.0 | 0.37 ± 0.07 | 0.27 ± 0.03 |
| guaia-3–9-diene | sesquiterpenes | 1413 | 1.08 ± 0.11 | 0.80 ± 0.09 | 1.60 ± 0.0 | 0.86 ± 0.1 | 0.00 ± 0.00 | 0.70 ± 0.09 |
| selina-3,7(11)-diene | sesquiterpenes | 1418 | 0.91 ± 0.08 | 0.48 ± 0.01 | 1.65 ± 0.1 | 0.80 ± 0.1 | 1.19 ± 0.21 | 0.47 ± 0.08 |
| caryophyllene oxide | sesquiterpenes | 1458 | 0.29 ± 0.04 | 0.27 ± 0.01 | 0.23 ± 0.0 | 0.29 ± 0.1 | 0.17 ± 0.03 | 0.20 ± 0.02 |
| cis-α-bisabolol | sesquiterpenes | 1589 | 0.05 ± 0.01 | 0.09 ± 0.07 | 0.04 ± 0.0 | 0.06 ± 0.00 | 0.32 ± 0.06 | 0.05 ± 0.00 |
| ID-Coriander Seeds | Terpenes Class | RI | N2h | N6h | S2h | S6h | UAE | M |
| α-pinene | bicyclic monoterpenes | 915 | 0.15 ± 0.04 | 0.33 ± 0.02 | 0.49 ± 0.07 | 0.15 ± 0.04 | 0.50 ± 0.01 | 0.34 ± 0.03 |
| 2-carene | bicyclic monoterpenes | 920 | 0.04 ± 0.01 | 0.07 ± 0.02 | 0.21 ± 0.01 | 0.04 ± 0.01 | 0.39 ± 0.01 | 0.04 ± 0.01 |
| p-mentha-1,3,8-triene | monocyclic monoterpenes | 980 | 0.04 ± 0.01 | 0.05 ± 0.01 | 1.40 ± 0.03 | 0.04 ± 0.01 | - | 0.49 ± 0.01 |
| β-terpinyl-acetate | monocyclic monoterpenes | 1343 | 0.04 ± 0.01 | 0.10 ± 0.00 | 0.04 ± 0.01 | 0.04 ± 0.01 | - | 0.13 ± 0.02 |
| eucaliptol | bicyclic monoterpenes | 935 | - | - | 0.24 ± 0.03 | - | 0.33 ± 0.00 | 0.03 ± 0.00 |
| β-myrcene | acyclic monoterpenes | 987 | 0.14 ± 0.03 | 0.21 ± 0.00 | 2.21 ± 0.01 | 0.15 ± 0.03 | 0.98 ± 0.01 | 0.23 ± 0.02 |
| β-ocymene | acyclic monoterpenes | 1027 | 0.05 ± 0.01 | 0.09 ± 0.01 | 0.02 ± 0.01 | 0.05 ± 0.01 | - | 0.04 ± 0.01 |
| ɣ-terpinene | monocyclic monoterpenes | 1053 | 0.15 ± 0.02 | 0.08 ± 0.01 | 2.24 ± 0.03 | 0.16 ± 0.02 | 1.41 ± 0.00 | 1.12 ± 0.01 |
| trans-linalool oxide | monocyclic monoterpenes | 1083 | 0.74 ± 0.03 | 0.59 ± 0.01 | 7.50 ± 0.04 | 0.75 ± 0.03 | 0.55 ± 0.04 | 2.09 ± 0.01 |
| terpinolene | monocyclic monoterpenes | 1085 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | - | 0.03 ± 0.01 |
| borneol | bicyclic monoterpenes | 1162 | 0.45 ± 0.03 | 0.39 ± 0.02 | 0.38 ± 0.06 | 0.45 ± 0.03 | - | 1.65 ± 0.01 |
| linalool | acyclic monoterpenes | 1079 | 84.16 ± 0.56 | 72.55 ± 0.03 | 54.73 ± 0.05 | 83.99 ± 0.56 | 82.95 ± 0.05 | 78.20 ± 0.02 |
| canfora | bicyclic monoterpenes | 1144 | 6.41 ± 0.05 | 3.26 ± 0.02 | 3.11 ± 0.45 | 6.47 ± 0.05 | 1.87 ± 0.03 | 7.90 ± 0.02 |
| terpinen-4-ol | monocyclic monoterpenes | 1173 | 0.66 ± 0.02 | 0.78 ± 0.00 | 6.05 ± 0.04 | 0.67 ± 0.02 | 0.47 ± 0.02 | 0.76 ± 0.01 |
| α-terpineol | monocyclic monoterpenes | 1190 | 1.05 ± 0.02 | 2.14 ± 0.03 | 13.30 ± 0.04 | 1.06 ± 0.02 | 5.65 ± 0.04 | 1.19 ± 0.01 |
| cis-geraniol | acyclic monoterpenes | 1248 | 3.96 ± 0.14 | 13.08 ± 0.02 | 0.25 ± 0.01 | 4.00 ± 0.14 | 0.67 ± 0.04 | 4.25 ± 0.04 |
| lavandulyl acetate | acyclic monoterpenes | 1270 | 1.42 ± 0.03 | 5.50 ± 0.01 | 0.77 ± 0.05 | 1.44 ± 0.03 | 1.44 ± 0.03 | 1.28 ± 0.02 |
| geranyl acetate | acyclic monoterpenes | 1372 | 0.48 ± 0.03 | 0.70 ± 0.02 | 2.77 ± 0.01 | 0.49 ± 0.03 | 0.58 ± 0.03 | 0.22 ± 0.01 |
| Thyme | ||||||
| N2h | N6h | S2h | S6h | UAE | M | |
| Gallic acid | 42.74 ± 0.50 d | 58.73 ± 0.90 b | 678.67 ± 0.40 a | 48.78 ± 0.32 c | - | 40.23 ± 0.89 e |
| p-OH-benzoic acid | 190.47 ± 1.02 b | 197.15 ± 0.89 a | - | 148.68 ± 0.98 c | 55.04 ± 0.95 d | 17.97 ± 1.02 e |
| Chlorogenic acid | 66.54 ± 0.96 c | 120.44 ± 0.75 a | 30.07 ± 0.89 d | 97.21 ± 0.98 b | - | - |
| Vanillic acid | - | - | - | - | 376.27 ± 0.56 a | - |
| Caffeic acid | - | - | - | - | 145.92 ± 0.85 a | - |
| Syringic acid | - | - | - | - | - | 159.69 ± 1.02 a |
| Ferulic acid | - | 139.24 ± 0.96b | - | 516.02 ± 0.84 a | - | - |
| Rosmarinic acid | 34201.41 ± 1.05 b | 33955.20 ± 1.02 c | 51686.96 ± 0.95 a | 31549.93 ± 1.25 d | 15049.48 ± 1.09 e | 261.55 ± 0.82 f |
| Luteolin | 2671.96 ± 1.10 c | 1554.86 ± 1.05 f | 1931.34 ± 0.95 d | 1704.21 ± 1.32 e | 4143.43 ± 0.65 b | 2099.47 ± 0.84 a |
| Apigenin | 6608.97 ± 1.15 b | 5309.40 ± 1.03 c | 2909.13 ± 1.01 e | 7618.77 ± 0.98 a | 4416.70 ± 0.87 d | - |
| Carvacrol | 3499.84 ± 1.15 b | 885.03 ± 1.02 d | 2873.81 ± 0.99 c | 5595.41 ± 1.05 a | - | 220.49 ± 0.98 e |
| Hemp | ||||||
| Gallic acid | - | 52.29 ± 0.98 c | 118.07 ± 0.32 b | 408.92 ± 0.63 a | 35.10 ± 0.98 d | 36.02 ± 0.65 d |
| p-OH-benzoic acid | - | 47.70 ± 0.75 c | 95.18 ± 0.95 a | 36.42 ± 0.35 d | - | 52.41 ± 0.96 b |
| Chlorogenic acid | - | - | - | - | - | - |
| Vanillic acid | - | - | - | - | - | - |
| Caffeic acid | - | - | 36.98 ± 0.48 b | - | 81.81 ± 0.91 a | - |
| Syringic acid | - | - | - | - | 57.28 ± 0.64 a | - |
| Ferulic acid | - | 247.77 ± 0.64 a | - | - | - | 96.70 ± 0.93 b |
| Rosmarinic acid | 259.56 ± 0.97 c | 27.09 ± 0.85 f | 206.30 ± 0.94 d | 152.06 ± 0.65 e | 514.33 ± 1.01 a | 328.21 ± 1.10 b |
| Luteolin | 1572.05 ± 1.04 a | 304.37 ± 1.10 e | 502.83 ± 0.95 d | 753.01 ± 0.84 c | 1384.09 ± 1.09 b | 127.67 ± 1.03 f |
| Apigenin | 72.99 ± 1.02 a | 51.43 ± 0.48 c | 35.77 ± 0.95 d | 54.22 ± 1.06 b | - | - |
| Carvacrol | - | - | - | - | - | - |
| Coriander Seeds | ||||||
| Gallic acid | 42.37 ± 0.98 b | 49.05 ± 0.65 a | 22.45 ± 0.35 d | 22.73 ± 0.36 d | - | 31.02 ± 0.39 c |
| p-OH-benzoic acid | 274.83 ± 0.95 a | 31.80 ± 0.98 d | - | 89.22 ± 0.84 b | - | 46.61 ± 0.91 c |
| Chlorogenic acid | 74.80 ± 0.67 e | 480.49 ± 0.92 b | 149.47 ± 0.41 c | 490.56 ± 0.35 a | 146.90 ± 0.83 d | 27.82 ± 0.94 f |
| Vanillic acid | 120.27 ± 0.87 e | 208.65 ± 0.80 c | 440.90 ± 1.25 a | 273.23 ± 1.65 b | 205.46 ± 0.96 d | - |
| Caffeic acid | - | 208.66 ± 0.85 b | 440.17 ± 0.75 a | 54.21 ± 0.65 c | 27.88 ± 0.35 d | - |
| Syringic acid | - | - | 24.09 ± 0.35 b | 65.20 ± 0.92 a | - | 23.56 ± 0.24 b |
| Ferulic acid | 188.92 ± 0.95 b | 78.81 ± 0.85 c | 20.64 ± 0.77 e | 239.21 ± 0.8 7a | 22.75 ± 0.64 d | 23.37 ± 0.81 d |
| Rosmarinic acid | 81.05 ± 1.05a | 82.13 ± 0.97a | 34.78 ± 0.91b | 31.00 ± 0.85c | 21.97 ± 0.98c | - |
| Luteolin | 324.50 ± 0.94a | 295.82 ± 1.12b | 172.40 ± 1.18c | 86.90 ± 0.98e | 152.01 ± 1.32d | - |
| Apigenin | 182.67 ± 1.20a | 182.04 ± 1.15a | 30.09 ± 1.06d | 35.81 ± 1.14b | 101.07 ± 1.23e | 87.31 ± 0.98c |
| Carvacrol | - | - | - | - | - | - |
| TPC (mg GAE/g Dry Extract) | FRAP (mg TE/g Dry Extract) | DPPH˙ (mg TE/g Dry Extract) | ABTS˙+ (mg TE/g Dry Extract) | |
|---|---|---|---|---|
| Thyme | ||||
| N2h | 141.86 ± 7.85 b | 63.86 ± 0.29 b | 108.17 ± 0.08 a | 23.62 ± 8.20 d |
| N6h | 179.67 ± 12.71 a | 66.95 ± 5.74 b | 108.52 ± 0.16 a | 52.12 ± 0.11 bc |
| S2h | 157.86 ± 1.71 b | 68.48 ± 1.23 ab | 108.57 ± 0.08 a | 49.07 ± 0.11 c |
| S6h | 154.26 ± 11.23 b | 73.53 ± 1.29 a | 107.19 ± 1.22 a | 134.46 ± 0.22 a |
| UAE | 107.91 ± 9.01 c | 40.60 ± 1.04 c | 98.50 ± 1.71 b | 53.72 ± 0.34 bc |
| M | 142.08 ± 1.69 b | 40.25 ± 2.93 c | 92.60 ± 4.33 c | 57.56 ± 0.34 b |
| Hemp | ||||
| N2h | 140.25 ± 2.56 a | 81.073 ± 2.31ab | 34.02 ± 1.86 b | 557.16 ± 6.57 a |
| N6h | 139.52 ± 2.49 a | 80.21 ± 1.76 abc | 33.76 ± 0.89 b | 485.10 ± 4.16 b |
| S2h | 120.55 ± 5.42 b | 74.66 ± 0.81 d | 29.92 ± 0.32 d | 394.39 ± 3.41 c |
| S6h | 124.25 ± 3.61 b | 78.69 ± 1.88 bc | 31.54 ± 0.08 cd | 433.22 ± 19.81 bc |
| UAE | 110.30 ± 3.71 c | 77.22 ± 0.92 cd | 45.04 ± 1.23 a | 381.26 ± 9.05 c d |
| M | 125.12 ± 3.54 b | 83.14 ± 1.63 a | 32.43 ± 0.32 bc | 502.16 ± 5.62 ab |
| Coriander Seeds | ||||
| N2h | 19.87 ± 1.52 b | 18.43 ± 0.15 a | 147.60 ± 3.97 b | 48.05 ± 1.60 a |
| N6h | 17.67 ± 0.47 c | 12.69 ± 0.39 d | 163.55 ± 2.94 b | 46.48 ± 1.78 a |
| S2h | 15.54 ± 0.57 d | 15.22 ± 0.39 bc | 128.84 ± 3.94 b | 21.90 ± 2.00 b |
| S6h | 24.36 ± 1.01 a | 16.91 ± 2.34 ab | 150.61± 4.92 b | 23.85 ± 1.60 b |
| UAE | 3.01 ± 0.61 e | 13.45 ± 1.26 cd | 219.95 ± 2.44 a | 1.12 ± 0.14 d |
| M | 18.95 ± 0.11 bc | 14.40 ± 0.09 cd | 177.23 ± 1.46 ab | 5.64 ± 1.04 c |
| TPC | FRAP | DPPH˙ | ABTS˙+ | |
|---|---|---|---|---|
| Thyme | N6h | S6h | N2h/N6h/S2h/S6h | S6h |
| Hemp | N2h/N6h | M | UAE | N2h |
| Coriander seeds | S6h | N2h | UAE | N2h/N6h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods 2020, 9, 1221. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091221
Palmieri S, Pellegrini M, Ricci A, Compagnone D, Lo Sterzo C. Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques. Foods. 2020; 9(9):1221. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091221
Chicago/Turabian StylePalmieri, Sara, Marika Pellegrini, Antonella Ricci, Dario Compagnone, and Claudio Lo Sterzo. 2020. "Chemical Composition and Antioxidant Activity of Thyme, Hemp and Coriander Extracts: A Comparison Study of Maceration, Soxhlet, UAE and RSLDE Techniques" Foods 9, no. 9: 1221. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9091221