Advancing Dentistry through Bioprinting: Personalization of Oral Tissues
Abstract
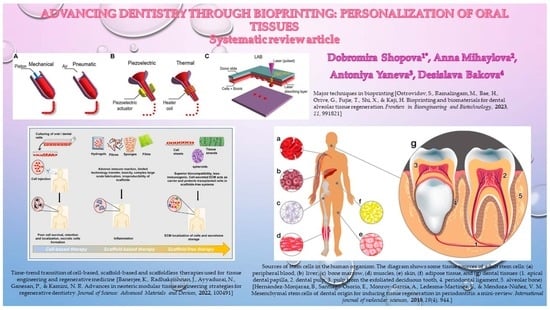
:1. Introduction
1.1. Scaffold-Free Bioprinting and Stem Cell Diversity in Dentistry
1.2. Dental Stem Cell Diversity and Regenerative Dentistry Prospects
2. Materials and Methods
2.1. Literature Search
2.2. Eligibility Criteria
2.3. Data Items
- Types of Bioprinting Applications in regenerative dentistry.
- Bioprinting Materials commonly used in bioprinting for regenerative dentistry, encompassing biocompatible polymers, bioinks, and scaffold materials.
- Bioprinting Techniques utilized in regenerative dentistry.
- Dental Tissues and Structures: Our review focuses on the specific dental tissues and structures that have been the main points of bioprinting applications, encompassing dentin, pulp, bone, periodontal tissues, and gingival soft tissues.
2.4. Data Analysis
3. Results
3.1. Dental Pulp-Dentin Regeneration
3.2. Bone Regeneration
3.3. Periodontium Regeneration
3.4. Gingival Regeneration
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ostrovidov, S.; Ramalingam, M.; Bae, H.; Orive, G.; Fujie, T.; Shi, X.; Kaji, H. Bioprinting and biomaterials for dental alveolar tissue regeneration. Front. Bioeng. Biotechnol. 2023, 11, 991821. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.G.; Tomlinson, R.E. Leveraging advancements in tissue engineering for bioprinting dental tissues. Bioprinting 2021, 23, e00153. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Zhang, P.; Liu, Y.; Lv, L.; Zhou, Y. Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomater. 2019, 101, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef]
- Banerjee, K.; Radhakrishnan, J.; Ayyadurai, N.; Ganesan, P.; Kamini, N.R. Advances in neoteric modular tissue engineering strategies for regenerative dentistry. J. Sci. Adv. Mater. Devices 2022, 7, 100491. [Google Scholar] [CrossRef]
- Salar Amoli, M.; EzEldeen, M.; Jacobs, R.; Bloemen, V. Materials for dentoalveolar bioprinting: Current state of the art. Biomedicines 2021, 10, 71. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental tissue-derived human mesenchymal stem cells and their potential in therapeutic application. Stem Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Monroy-García, A.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal stem cells of dental origin for inducing tissue regeneration in periodontitis: A mini-review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Norotte, C.; Marga, F.S.; Niklason, L.E.; Forgacs, G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009, 30, 5910–5917. [Google Scholar] [CrossRef] [PubMed]
- Munaz, A.; Vadivelu, R.K.; St John, J.; Barton, M.; Kamble, H.; Nguyen, N.-T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Tao, O.; Kort-Mascort, J.; Lin, Y.; Pham, H.M.; Charbonneau, A.M.; ElKashty, O.A.; Tran, S.D. The applications of 3D printing for craniofacial tissue engineering. Micromachines 2019, 10, 480. [Google Scholar] [CrossRef]
- Morsczeck, C.; Schmalz, G.; Reichert, T.E.; Völlner, F.; Galler, K.; Driemel, O. Somatic stem cells for regenerative dentistry. Clin. Oral Investig. 2008, 12, 113–118. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Pierdomenico, L.; Bonsi, L.; Calvitti, M.; Rondelli, D.; Arpinati, M.; Chirumbolo, G.; Bagnara, G.P. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 2005, 80, 836–842. [Google Scholar] [CrossRef]
- La Noce, M.; Paino, F.; Spina, A.; Naddeo, P.; Montella, R.; Desiderio, V.; Laino, L. Dental pulp stem cells: State of the art and suggestions for a true translation of research into therapy. J. Dent. 2014, 42, 761–768. [Google Scholar] [CrossRef]
- Yasui, T.; Mabuchi, Y.; Toriumi, H.; Ebine, T.; Niibe, K.; Houlihan, D.D.; Matsuzaki, Y. Purified human dental pulp stem cells promote osteogenic regeneration. J. Dent. Res. 2016, 95, 206–214. [Google Scholar] [CrossRef]
- D’Aquino, R.; Papaccio, G.; Laino, G. Dental Pulp Stem Cells: A Promising Tool for Bone Regeneration. Stem Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Collart-Dutilleul, P.Y.; Chaubron, F.; De Vos, J.; Cuisinier, F.J. Allogenic banking of dental pulp stem cells for innovative therapeutics. World J. Stem Cells 2015, 7, 1010. [Google Scholar] [PubMed]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sha, X.J.; Li, G.H.; Yang, F.S.; Ji, K.; Wen, L.Y.; Xuan, K. Comparative characterization of stem cells from human exfoliated deciduous teeth and dental pulp stem cells. Arch. Oral Biol. 2012, 57, 1231–1240. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Karbanová, J.; Soukup, T.; Suchánek, J.; Pytlík, R.; Corbeil, D.; Mokrý, J. Characterization of dental pulp stem cells from impacted third molars cultured in low serum-containing medium. Cells Tissues Organs 2011, 193, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Mori, G.; Ballini, A.; Carbone, C.; Oranger, A.; Brunetti, G.; Di Benedetto, A.; Grassi, F.R. Osteogenic differentiation of dental follicle stem cells. Int. J. Med. Sci. 2012, 9, 480. [Google Scholar] [CrossRef]
- Honda, M.J.; Imaizumi, M.; Tsuchiya, S.; Morsczeck, C. Dental follicle stem cells and tissue engineering. J. Oral Sci. 2010, 52, 541–552. [Google Scholar] [CrossRef]
- Yao, S.; Pan, F.; Prpic, V.; Wise, G.E. Differentiation of stem cells in the dental follicle. J. Dent. Res. 2008, 87, 767–771. [Google Scholar] [CrossRef]
- Handa, K.; Saito, M.; Tsunoda, A.; Yamauchi, M.; Hattori, S.; Sato, S.; Narayanan, A.S. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect. Tissue Res. 2002, 43, 406–408. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Nakahara, T.; Ishikawa, H.; Sato, S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology 2013, 101, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Yang, X. Mesenchymal stem cells and bone regeneration: Current status. Injury 2011, 42, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Ojansivu, M.; van der Koog, L.; Whittaker, T.E.; Cunnane, E.M.; Silva, A.M.; Stevens, M.M. Extracellular vesicles for tissue repair and regeneration: Evidence, challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 175, 113775. [Google Scholar] [CrossRef]
- Lai, H.; Li, J.; Kou, X.; Mao, X.; Zhao, W.; Ma, L. Extracellular vesicles for dental pulp and periodontal regeneration. Pharmaceutics 2023, 15, 282. [Google Scholar] [CrossRef]
- Abbass, M.M.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Fawzy El-Sayed, K.M. Hydrogels and dentin–pulp complex regeneration: From the benchtop to clinical translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Lin, Z. Functional dental pulp regeneration: Basic research and clinical translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Ayala-Ham, A.; López-Gutierrez, J.; Bermúdez, M.; Aguilar-Medina, M.; Sarmiento-Sánchez, J.I.; López-Camarillo, C.; Ramos-Payan, R. Hydrogel-based scaffolds in oral tissue engineering. Front. Mater. 2021, 8, 708945. [Google Scholar] [CrossRef]
- Sugiaman, V.K.; Jeffrey Naliani, S.; Pranata, N.; Djuanda, R.; Saputri, R.I. Polymeric Scaffolds Used in Dental Pulp Regeneration by Tissue Engineering Approach. Polymers 2023, 15, 1082. [Google Scholar] [CrossRef]
- Gao, X.; Qin, W.; Wang, P.; Wang, L.; Weir, M.D.; Reynolds, M.A.; Xu, H.H. Nano-structured demineralized human dentin matrix to enhance bone and dental repair and regeneration. Appl. Sci. 2019, 9, 1013. [Google Scholar] [CrossRef]
- Ohlsson, E.; Galler, K.M.; Widbiller, M. A compilation of study models for dental pulp regeneration. Int. J. Mol. Sci. 2022, 23, 14361. [Google Scholar] [CrossRef] [PubMed]
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in Regenerative Dentistry Approaches: An Update. Int. Dent. J. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, M.; Yue, L.; Huang, D.; Zhou, X.; Wang, X.; Ling, J. Expert consensus on regenerative endodontic procedures. Int. J. Oral Sci. 2022, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Bertassoni, L.E. Progress and challenges in microengineering the dental pulp vascular microenvironment. J. Endod. 2020, 46, S90–S100. [Google Scholar] [CrossRef]
- Widbiller, M.; Galler, K.M. Engineering the Future of Dental Health: Exploring Molecular Advancements in Dental Pulp Regeneration. Int. J. Mol. Sci. 2023, 24, 11453. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect. Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef]
- França, C.M.; Sercia, A.; Parthiban, S.P.; Bertassoni, L.E. Current and Future Applications of 3D Bioprinting in Endodontic Regeneration—A Short Review. J. Calif. Dent. Assoc. 2019, 47, 645–652. [Google Scholar] [CrossRef]
- França, C.M.; Riggers, R.; Muschler, J.L.; Widbiller, M.; Lococo, P.M.; Diogenes, A.; Bertassoni, L.E. 3D-imaging of whole neuronal and vascular networks of the human dental pulp via CLARITY and light sheet microscopy. Sci. Rep. 2019, 9, 10860. [Google Scholar] [CrossRef]
- Tian, T.; Yang, Z.; Li, X. Tissue clearing technique: Recent progress and biomedical applications. J. Anat. 2021, 238, 489–507. [Google Scholar] [CrossRef]
- Parthiban, S.P.; He, W.; Monteiro, N.; Athirasala, A.; França, C.M.; Bertassoni, L.E. Engineering pericyte-supported microvascular capillaries in cell-laden hydrogels using stem cells from the bone marrow, dental pulp and dental apical papilla. Sci. Rep. 2020, 10, 21579. [Google Scholar] [CrossRef]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Khoury, M. Cell-based regenerative endodontics for treatment of periapical lesions: A randomized, controlled phase I/II clinical trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a New Concept of Regenerative Endodontics Based on Mesenchymal Stem Cell-Derived Secretomes Products. Bioengineering 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Dolega-Dolegowski, D.; Dolega-Dolegowska, M.; Pregowska, A.; Malinowski, K.; Proniewska, K. The application of mixed reality in root canal treatment. Appl. Sci. 2023, 13, 4078. [Google Scholar] [CrossRef]
- Iandolo, A. Modern Advances in Microendodontics: The State of the Art. Bioengineering 2023, 10, 789. [Google Scholar] [CrossRef]
- Arshad, S.; Tehreem, F.; Ahmed, F.; Marya, A.; Karobari, M.I. Platelet-rich fibrin used in regenerative endodontics and dentistry: Current uses, limitations, and future recommendations for application. Int. J. Dent. 2021, 2021, 4514598. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lin, Y.; Xu, X.; Chen, Z.; Xiang, Y.; Yang, L.; Chen, X. Clinical observation of autologous platelet rich fibrin assisted revascularization of mature permanent teeth. Head Face Med. 2023, 19, 9. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jin, H.; Lin, S.; Ma, L.; Tian, T.; Qin, X. Are platelet concentrate scaffolds superior to traditional blood clot scaffolds in regeneration therapy of necrotic immature permanent teeth? A systematic review and meta-analysis. BMC Oral Health 2022, 22, 589. [Google Scholar] [CrossRef]
- Heboyan, A.; Avetisyan, A.; Karobari, M.I.; Marya, A.; Khurshid, Z.; Rokaya, D.; Fernandes, G.V.D.O. Tooth root resorption: A review. Sci. Prog. 2022, 105, 00368504221109217. [Google Scholar] [CrossRef]
- Soares, D.G.; Bordini, E.A.; Swanson, W.B.; de Souza Costa, C.A.; Bottino, M.C. Platform technologies for regenerative endodontics from multifunctional biomaterials to tooth-on-a-chip strategies. Clin. Oral Investig. 2021, 25, 4749–4779. [Google Scholar] [CrossRef]
- Bahraminasab, M.; Janmohammadi, M.; Arab, S.; Talebi, A.; Nooshabadi, V.T.; Koohsarian, P.; Nourbakhsh, M.S. Bone scaffolds: An incorporation of biomaterials, cells, and biofactors. ACS Biomater. Sci. Eng. 2021, 7, 5397–5431. [Google Scholar] [CrossRef] [PubMed]
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.P. Challenges in bone tissue regeneration: Stem cell therapy, biofunctionality and antimicrobial properties of novel materials and its evolution. Int. J. Mol. Sci. 2020, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Kirankumar, S.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Vasanthan, J.; Perales, S.G.; Rajasingh, J. Modern approaches on stem cells and scaffolding technology for osteogenic differentiation and regeneration. Exp. Biol. Med. 2022, 247, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Geng, Y.-M.; Li, S.-Y.; Yang, X.-B.; Che, Y.-J.; Pathak, J.L.; Wu, G. Nanocrystalline hydroxyapatite-based scaffold adsorbs and gives sustained release of osteoinductive growth factor and facilitates bone regeneration in mice ectopic model. J. Nanomater. 2019, 2019, 10. [Google Scholar] [CrossRef]
- Liu, J.; Ruan, J.; Weir, M.D.; Ren, K.; Schneider, A.; Wang, P.; Xu, H.H. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells 2019, 8, 537. [Google Scholar] [CrossRef]
- Su, X.; Wang, T.; Guo, S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 2021, 16, 63–72. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Gowdini, M.; Khademi, A.; Dehghani, M.; Latifi, M.; Alsaadi, N.; Khan, A. Bioprinting of three-dimensional scaffold based on alginate-gelatin as soft and hard tissue regeneration. J. Mater. Res. Technol. 2021, 14, 2853–2864. [Google Scholar] [CrossRef]
- Sordi, M.B.; Cruz, A.; Fredel, M.C.; Magini, R.; Sharpe, P.T. Three-dimensional bioactive hydrogel-based scaffolds for bone regeneration in implant dentistry. Mater. Sci. Eng. C 2021, 124, 112055. [Google Scholar] [CrossRef]
- Li, W.; Wu, Y.; Zhang, X.; Wu, T.; Huang, K.; Wang, B.; Liao, J. Self-healing hydrogels for bone defect repair. RSC Adv. 2023, 13, 16773–16788. [Google Scholar] [CrossRef]
- Salah, M.; Tayebi, L.; Moharamzadeh, K.; Naini, F.B. Three-dimensional bio-printing and bone tissue engineering: Technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac. Plast. Reconstr. Surg. 2020, 42, 1–9. [Google Scholar] [CrossRef]
- Khorsandi, D.; Fahimipour, A.; Abasian, P.; Saber, S.S.; Seyedi, M.; Ghanavati, S.; Makvandi, P. 3D and 4D printing in dentistry and maxillofacial surgery: Printing techniques, materials, and applications. Acta Biomater. 2021, 122, 26–49. [Google Scholar] [CrossRef]
- Lim, H.K.; Hong, S.J.; Byeon, S.J.; Chung, S.M.; On, S.W.; Yang, B.E.; Byun, S.H. 3D-printed ceramic bone scaffolds with variable pore architectures. Int. J. Mol. Sci. 2020, 21, 6942. [Google Scholar] [CrossRef]
- Son, C.; Choi, M.S.; Park, J. Different Responsiveness of Alveolar Bone and Long Bone to Epithelial-Mesenchymal Interaction-Related Factor. JBMR Plus 2020, 4, e10382. [Google Scholar] [CrossRef] [PubMed]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and Synthetic Polymers for Bone Scaffolds Optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xu, Y.; Zhang, T.; Ma, Y.; Liu, J.; Yuan, B.; Chen, X.; Zhou, P.; Zhao, X.; Pang, F. Mesenchymal stem cell sheets: A new cell-based strategy for bone repair and regeneration. Biotechnol. Lett. 2019, 41, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Imashiro, C.; Shimizu, T. Fundamental technologies and recent advances of cell-sheet-based tissue engineering. Int. J. Mol. Sci. 2021, 22, 425. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Zurina, I.M.; Presniakova, V.S.; Kosheleva, N.V.; Butnaru, D.V.; Svistunov, A.A.; Timashev, P.S. Mechanical properties of cell sheets and spheroids: The link between single cells and complex tissues. Biophys. Rev. 2021, 13, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Hasani-Sadrabadi, M.M.; Sarrion, P.; Pouraghaei, S.; Chau, Y.; Ansari, S.; Li, S.; Moshaverinia, A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci. Transl. Med. 2020, 12, eaay6853. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug Deliv. Sci. Technol. 2021, 64, 102600. [Google Scholar] [CrossRef]
- Gao, Q.; Kim, B.S.; Gao, G. Advanced strategies for 3D bioprinting of tissue and organ analogs using alginate hydrogel bioinks. Mar. Drugs 2021, 19, 708. [Google Scholar] [CrossRef]
- Lin, H.; Tang, Y.; Lozito, T.P.; Oyster, N.; Wang, B.; Tuan, R.S. Efficient in vivo bone formation by BMP-2 engineered human mesenchymal stem cells encapsulated in a projection stereolithographically fabricated hydrogel scaffold. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Vurat, M.T.; Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Bioactive composite hydrogels as 3D mesenchymal stem cell encapsulation environment for bone tissue engineering: In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2023, 111, 261–277. [Google Scholar] [CrossRef]
- Van Hede, D.; Liang, B.; Anania, S.; Barzegari, M.; Verlée, B.; Nolens, G.; Lambert, F. 3D-Printed Synthetic Hydroxyapatite Scaffold With In Silico Optimized Macrostructure Enhances Bone Formation In Vivo. Adv. Funct. Mater. 2022, 32, 2105002. [Google Scholar] [CrossRef]
- Dwivedi, R.; Mehrotra, D. 3D bioprinting and craniofacial regeneration. J. Oral Biol. Craniofacial Res. 2020, 10, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Atia, G.A.N.; Shalaby, H.K.; Ali, N.G.; Morsy, S.M.; Ghobashy, M.M.; Attia, H.A.N.; Barai, H.R. New Challenges and Prospective Applications of Three-Dimensional Bioactive Polymeric Hydrogels in Oral and Craniofacial Tissue Engineering: A Narrative Review. Pharmaceuticals 2023, 16, 702. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for biomedical applications: A short overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Zheng, L. Calcium Phosphate-Based Biomaterials for Bone Repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Anderson, M.; Dubey, N.; Bogie, K.; Cao, C.; Li, J.; Lerchbacker, J.; Kaigler, D. Three-dimensional printing of clinical scale and personalized calcium phosphate scaffolds for alveolar bone reconstruction. Dent. Mater. 2022, 38, 529–539. [Google Scholar] [CrossRef]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 137, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liang, B.; Jiang, H.; Deng, Z.; Yu, K. Magnesium-based biomaterials as emerging agents for bone repair and regeneration: From mechanism to application. J. Magnes. Alloys 2021, 9, 779–804. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Koc, M. Additive manufacturing of biodegradable magnesium implants and scaffolds: Review of the recent advances and research trends. J. Magnes. Alloys 2021, 9, 392–415. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wang, M.; Backman, L.J.; Chen, J. Effects of zinc, magnesium, and iron ions on bone tissue engineering. ACS Biomater. Sci. Eng. 2022, 8, 2321–2335. [Google Scholar] [CrossRef] [PubMed]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Zarrabi, A.; Afghah, F.; Koc, B.; Adibkia, K. Effect of zinc-doped hydroxyapatite/graphene nanocomposite on the physicochemical properties and osteogenesis differentiation of 3D-printed polycaprolactone scaffolds for bone tissue engineering. Chem. Eng. J. 2021, 426, 131321. [Google Scholar] [CrossRef]
- Samiei, M.; Alipour, M.; Khezri, K.; Saadat, Y.R.; Forouhandeh, H.; Abdolahinia, E.D.; Dizaj, S.M. Application of collagen and mesenchymal stem cells in regenerative dentistry. Curr. Stem Cell Res. Ther. 2022, 17, 606–620. [Google Scholar]
- Tharakan, S.; Khondkar, S.; Lee, S.; Ahn, S.; Mathew, C.; Gresita, A.; Ilyas, A. 3D Printed Osteoblast–Alginate/Collagen Hydrogels Promote Survival, Proliferation and Mineralization at Low Doses of Strontium Calcium Polyphosphate. Pharmaceutics 2022, 15, 11. [Google Scholar] [CrossRef]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as bioink for bioprinting: A comprehensive review. Int. J. Bioprinting 2020, 6, 270. [Google Scholar] [CrossRef]
- Wang, H. A review of the effects of collagen treatment in clinical studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Zhao, X.; Pakvasa, M.; Tucker, A.B.; Luo, H.; El Dafrawy, M. Stem cell-friendly scaffold biomaterials: Applications for bone tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 2020, 8, 598607. [Google Scholar] [CrossRef]
- Zhang, J.; Wehrle, E.; Adamek, P.; Paul, G.R.; Qin, X.H.; Rubert, M.; Müller, R. Optimization of mechanical stiffness and cell density of 3D bioprinted cell-laden scaffolds improves extracellular matrix mineralization and cellular organization for bone tissue engineering. Acta Biomater. 2020, 114, 307–322. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; Mazzoni, E.; Bononi, I.; Rotondo, J.C.; Mazziotta, C.; Montesi, M.; Martini, F. Adult stem cells for bone regeneration and repair. Front. Cell Dev. Biol. 2019, 7, 268. [Google Scholar] [CrossRef] [PubMed]
- Loukelis, K.; Helal, Z.A.; Mikos, A.G.; Chatzinikolaidou, M. Nanocomposite bioprinting for tissue engineering applications. Gels 2023, 9, 103. [Google Scholar] [CrossRef]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, L.; Csöbönyeiová, M. Stem Cells and Their Derivatives—Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology scaffolds for alveolar bone regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Tabei, Y. Role of oxidative stress in nanoparticles toxicity. Free Radic. Res. 2021, 55, 331–342. [Google Scholar] [CrossRef]
- Marew, T.; Birhanu, G. Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021, 18, 102–111. [Google Scholar] [CrossRef]
- Wang, N.; Dheen, S.T.; Fuh, J.Y.H.; Kumar, A.S. A review of multi-functional ceramic nanoparticles in 3D printed bone tissue engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Maia, F.R.; Bastos, A.R.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Recent approaches towards bone tissue engineering. Bone 2022, 154, 116256. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Burdis, R.; Kelly, D.J. Biofabrication and bioprinting using cellular aggregates, microtissues and organoids for the engineering of musculoskeletal tissues. Acta Biomater. 2021, 126, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Chen, Y.; Luo, J.; Liu, X.; Yang, Q.; Shi, X.; Wang, Y. Black phosphorus nanosheets-enabled DNA hydrogel integrating 3D-printed scaffold for promoting vascularized bone regeneration. Bioact. Mater. 2023, 21, 97–109. [Google Scholar] [CrossRef]
- Tharakan, S.; Khondkar, S.; Ilyas, A. Bioprinting of stem cells in multimaterial scaffolds and their applications in bone tissue engineering. Sensors 2021, 21, 7477. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Trivedi, R. 3D printed bioconstructs: Regenerative modulation for genetic expression. Stem Cell Rev. Rep. 2021, 17, 1239–1250. [Google Scholar] [CrossRef]
- Alizadeh, P.; Soltani, M.; Tutar, R.; Hoque Apu, E.; Maduka, C.V.; Unluturk, B.D.; Ashammakhi, N. Use of electroconductive biomaterials for engineering tissues by 3D printing and 3D bioprinting. Essays Biochem. 2021, 65, 441–466. [Google Scholar]
- Ausenda, F.; Rasperini, G.; Acunzo, R.; Gorbunkova, A.; Pagni, G. New perspectives in the use of biomaterials for periodontal regeneration. Materials 2019, 12, 2197. [Google Scholar] [CrossRef]
- Nicholson, J.W. Periodontal Therapy Using Bioactive Glasses: A Review. Prosthesis 2022, 4, 648–663. [Google Scholar] [CrossRef]
- Vahdatinia, F.; Hooshyarfard, A.; Jamshidi, S.; Shojaei, S.; Patel, K.; Moeinifard, E.; Tayebi, L. 3D-Printed Soft Membrane for Periodontal Guided Tissue Regeneration. Materials 2023, 16, 1364. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Alauddin, M.S.; Abdul Hayei, N.A.; Sabarudin, M.A.; Mat Baharin, N.H. Barrier membrane in regenerative therapy: A narrative review. Membranes 2022, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next-generation surgical meshes for drug delivery and tissue engineering applications: Materials, design and emerging manufacturing technologies. Bio-Des. Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Raveau, S.; Jordana, F. Tissue Engineering and Three-Dimensional Printing in Periodontal Regeneration: A Literature Review. J. Clin. Med. 2020, 9, 4008. [Google Scholar] [CrossRef] [PubMed]
- Sufaru, I.G.; Macovei, G.; Stoleriu, S.; Martu, M.A.; Luchian, I.; Kappenberg-Nitescu, D.C.; Solomon, S.M. 3D Printed and Bioprinted Membranes and Scaffolds for the Periodontal Tissue Regeneration: A Narrative Review. Membranes 2022, 12, 902. [Google Scholar] [CrossRef]
- D’Avanzo, N.; Bruno, M.C.; Giudice, A.; Mancuso, A.; Gaetano, F.D.; Cristiano, M.C.; Fresta, M. Influence of materials properties on bio-physical features and effectiveness of 3D-scaffolds for periodontal regeneration. Molecules 2021, 26, 1643. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.N.; Cho, Y.J.; Tarafder, S.; Lee, C.H. The recent advances in scaffolds for integrated periodontal regeneration. Bioact. Mater. 2021, 6, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Bousnaki, M.; Beketova, A.; Kontonasaki, E. A review of in vivo and clinical studies applying scaffolds and cell sheet technology for periodontal ligament regeneration. Biomolecules 2022, 12, 435. [Google Scholar] [CrossRef]
- Yang, X.; Ma, Y.; Wang, X.; Yuan, S.; Huo, F.; Yi, G.; Tian, W. A 3D-Bioprinted Functional Module Based on Decellularized Extracellular Matrix Bioink for Periodontal Regeneration. Adv. Sci. 2023, 10, 2205041. [Google Scholar] [CrossRef]
- Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. [Google Scholar] [CrossRef]
- Miao, G.; Liang, L.; Li, W.; Ma, C.; Pan, Y.; Zhao, H.; Yang, X. 3D Bioprinting of a Bioactive Composite Scaffold for Cell Delivery in Periodontal Tissue Regeneration. Biomolecules 2023, 13, 1062. [Google Scholar] [CrossRef]
- Hsieh, H.Y.; Yao, C.C.; Hsu, L.F.; Tsai, L.H.; Jeng, J.H.; Young, T.H.; Chen, Y.J. Biological properties of human periodontal ligament cell spheroids cultivated on chitosan and polyvinyl alcohol membranes. J. Formos. Med. Assoc. 2022, 121, 2191–2202. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, T.; Dieterle, M.P.; Tomakidi, P. Molecular research on oral diseases and related biomaterials: A journey from oral cell models to advanced regenerative perspectives. Int. J. Mol. Sci. 2022, 23, 5288. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, J.Y.; Bhattarai, G.; So, H.S.; Kook, S.H.; Lee, J.C. Periodontal Ligament-Mimetic Fibrous Scaffolds Regulate YAP-Associated Fibroblast Behaviors and Promote Regeneration of Periodontal Defect in Relation to the Scaffold Topography. ACS Appl. Mater. Interfaces 2022, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Kieu, T.T.T.; Kook, S.H.; Lee, J.C. Structurally optimized electrospun scaffold for biomaterial-controlled synergistic enhancement of defective bone healing. Smart Mater. Med. 2023, 4, 603–620. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Daghrery, A.; Aytac, Z.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Highly tunable bioactive fiber-reinforced hydrogel for guided bone regeneration. Acta Biomater. 2020, 113, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, T.; Sun, M.; Cheng, Z.; Jia, W.; Jiao, K.; Luo, Y. ZIF-8 modified multifunctional injectable photopolymerizable GelMA hydrogel for the treatment of periodontitis. Acta Biomater. 2022, 146, 37–48. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A review on the design of hydrogels with different stiffness and their effects on tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; Ferreira, J.A.; Xu, J.; Golafshan, N.; Kaigler, D.; Bhaduri, S.B.; Bottino, M.C. Tissue-specific melt electrowritten polymeric scaffolds for coordinated regeneration of soft and hard periodontal tissues. Bioact. Mater. 2023, 19, 268–281. [Google Scholar] [CrossRef]
- Daghrery, A.; Bottino, M.C. Advanced biomaterials for periodontal tissue regeneration. Genesis 2022, 60, e23501. [Google Scholar] [CrossRef]
- Xu, X.Y.; Li, X.; Wang, J.; He, X.T.; Sun, H.H.; Chen, F.M. Concise review: Periodontal tissue regeneration using stem cells: Strategies and translational considerations. Stem Cells Transl. Med. 2019, 8, 392–403. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Y.; Zheng, K.; Li, X.; Li, L.; Xu, Y. 3D Polycaprolactone/Gelatin-Oriented Electrospun Scaffolds Promote Periodontal Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 46145–46160. [Google Scholar] [CrossRef]
- Mancini, L.; Romandini, M.; Fratini, A.; Americo, L.M.; Panda, S.; Marchetti, E. Biomaterials for periodontal and peri-implant regeneration. Materials 2021, 14, 3319. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.; Li, J.; Ding, M.; Zhou, N.; Dong, H.; Mou, Y. Stem cell therapies for periodontal tissue regeneration: A network meta-analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 427. [Google Scholar] [CrossRef]
- Nagayasu-Tanaka, T.; Anzai, J.; Takedachi, M.; Kitamura, M.; Harada, T.; Murakami, S. Effects of combined application of fibroblast growth factor (FGF)-2 and carbonate apatite for tissue regeneration in a beagle dog model of one-wall periodontal defect. Regen. Ther. 2023, 23, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ardhani, R.; Hafiyyah, O.A.; Fauzi, M.B. Current Insight of Collagen Biomatrix for Gingival Recession: An Evidence-Based Systematic Review. Polymers 2020, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Ratanavaraporn, J.; Fauzi, M.B. Comprehensive review of hybrid collagen and silk fibroin for cutaneous wound healing. Materials 2020, 13, 3097. [Google Scholar] [CrossRef]
- Teerdha, P.D.; Admali, M.; Smriti, K.; Pentapati, K.C.; Vineetha, R.; Gadicherla, S. 3D bio-printing—A review on current application and future prospects in dentistry. J. Int. Dent. Med. Res. 2019, 12, 1202–1210. [Google Scholar]
- Dieterle, M.P.; Husari, A.; Steinberg, T.; Wang, X.; Ramminger, I.; Tomakidi, P. From the matrix to the nucleus and back: Mechanobiology in the light of health, pathologies, and regeneration of oral periodontal tissues. Biomolecules 2021, 11, 824. [Google Scholar] [CrossRef]
- Liao, W.; Xu, L.; Wangrao, K.; Du, Y.; Xiong, Q.; Yao, Y. Three-dimensional printing with biomaterials in craniofacial and dental tissue engineering. PeerJ 2019, 7, e7271. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Willems, G.; He, H.; Cadenas de Llano-Pérula, M. Nanomaterials for Periodontal Tissue Regeneration: Progress, Challenges and Future Perspectives. J. Funct. Biomater. 2023, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Hutomo, D.I.; Amir, L.; Suniarti, D.F.; Bachtiar, E.W.; Soeroso, Y. Hydrogel-Based Biomaterial as a Scaffold for Gingival Regeneration: A Systematic Review of In Vitro Studies. Polymers 2023, 15, 2591. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Feng, Y. Chitosan Hydrogel as Tissue Engineering Scaffolds for Vascular Regeneration Applications. Gels 2023, 9, 373. [Google Scholar] [CrossRef]
- Janowicz, K.; Mozdziak, P.; Bryja, A.; Kempisty, B.; Dyszkiewicz-Konwińska, M. Human dental pulp stem cells: Recent findings and current research. Med. J. Cell Biol. 2019, 7, 119–124. [Google Scholar] [CrossRef]
- Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules 2021, 11, 997. [Google Scholar] [CrossRef]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Zhou, X. Dental follicle cells: Roles in development and beyond. Stem Cells Int. 2019, 2019, 9159605. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liao, L.; Tian, W. Stem cell-based dental pulp regeneration: Insights from signaling pathways. Stem Cell Rev. Rep. 2021, 17, 1251–1263. [Google Scholar] [CrossRef]
- Tiozzo-Lyon, P.; Andrade, M.; Leiva-Sabadini, C.; Morales, J.; Olivares, A.; Ravasio, A.; Aguayo, S. Microfabrication approaches for oral research and clinical dentistry. Front. Dent. Med. 2023, 4, 1120394. [Google Scholar] [CrossRef]
- Baranova, J.; Büchner, D.; Götz, W.; Schulze, M.; Tobiasch, E. Tooth formation: Are the hardest tissues of human body hard to regenerate? Int. J. Mol. Sci. 2020, 21, 4031. [Google Scholar] [CrossRef]
- Kim, S.; Hwangbo, H.; Chae, S.; Lee, H. Biopolymers and Their Application in Bioprinting Processes for Dental Tissue Engineering. Pharmaceutics 2023, 15, 2118. [Google Scholar] [CrossRef]
- Ahmed, G.M.; Abouauf, E.A.; AbuBakr, N.; Dörfer, C.E.; El-Sayed, K.F. Tissue engineering approaches for enamel, dentin, and pulp regeneration: An update. Stem Cells Int. 2020, 2020, 5734539. [Google Scholar] [CrossRef] [PubMed]
- Tauqir, S.; Ali, S.; Marya, A. The Use of Bio-Inks and the Era of Bioengineering and Tooth Regeneration. Pesqui. Bras. Odontoped. Clínica Integr. 2022, 22, e210184. [Google Scholar] [CrossRef]





| Key Benefit/Topic | Area of Application/Significance | References |
|---|---|---|
| Dental pulp-dentin regeneration | 3D bioprinted scaffolds have potential to stimulate the differentiation of resident or transplanted stem/progenitor cells to regenerate the dentin-pulp complex | Abbass et al., 2020; Xie et al., 2021; Ayala-ham et al., 2021; Sugiaman et al., 2023 [37,38,39,40] |
| Realm of dentin, pulp, and periodontal regeneration using Demineralized dentin matrix | Gao et al., 2019 [41] | |
| Tissue engineering approaches offer pulp revascularization as an alternative by preserving tooth function. | Thalakiriyawa& Dissanayaka, 2023; Wei et al., 2022; Bertassoni, 2020; Widbiller & Galler, 2023 [43,44,45,46] | |
| Bbioprinted cell-loaded collagen-based bioinks showcases potential for root canal vasculogenesis | Campos et al., 2020 [47] | |
| 3D bioprinted materials conduce to dentin-pulp regeneration | Brizuela et al., 2020; Costa et al., 2022; Dolega-Dolegowski et al., 2023; Iandolo, 2023; Arshad et al., 2021; Wu et al., 2023; Tang et al., 2022; Heboyan et al., 2022 [52,53,54,55,56,57,58,59] | |
| Bone regeneration | Hydrogel based on alginate facilitated the regeneration of bone around dental implants | Hasani-Sadrabadi et al., 2020 [79] |
| Bioinks and hydrogels have the potential for the restoration of damaged dentin and pulp tissues | Samiei et al., 2021; Gao et al., 2021; Lin et al., 2019; Vurat et al., 2023; Van Hede et al., 2022 [80,81,82,83,84] | |
| 3D bioprinting platforms have the potential for fabricating craniofacial bone and cartilage structures | Dwivedi & Mehrotra, 2020; Atia et al., 2023 [85,86] | |
| Biomaterials for of scaffold that are conducive to cellular adherence and proliferation, have potential to lead addressing extensive bone defects | Zhang, et al., 2020; Zhang, et al., 2020; Iaquinta et al., 2019; Loukelis et al., 2023; Gan et al., 2020 [10,100,101,102,103] | |
| Periodontium regeneration | Three-dimensional printing of scaffolds has emerged as a compelling alternative to traditional periodontal regeneration methods | Raveau & Jordana, 2020; Sufaru et al., 2022; d’Avanzo et al., 2021; Woo et al., 2021; Bousnaki et al., 2022; Yang et al., 2023; Liang et al., 2023; Miao et al., 2023 [123,124,125,126,127,128,129,130] |
| Biomaterial-based approaches offer a comparably straightforward and reliably supportive means for substantial endogenous tissue regeneration | Xu et al., 2019; Matichescu et al., 2020 [140,141] | |
| Specific biomaterials have potential for new bone regeneration, and also for the emergence of angular, concentrated fiber regeneration on the root surface of the defect | Xu et al., 2020 [142] | |
| Bioactive factors influence the differentiation of precursor cells into specific periodontal tissues, stimulate resident stem cells to migrate to damaged sites, and attract immune cells to modulate the inflammatory response | Xu et al., 2019; Mancini et al., 2021; Liu et al., 2020; Zhai et al., 2019 [17,140,143,144] | |
| Gingival regeneration | The application of nanomaterials could potentially alter the gingiva’s color, shape, and texture, significantly impacting smile aesthetics, especially in the context of anterior teeth | Zong et al., 2023 [151] |
| Hydrogel materials are emerging as promising scaffold biomaterials for gingival regeneration | Hutomo et al., 2023 [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shopova, D.; Mihaylova, A.; Yaneva, A.; Bakova, D. Advancing Dentistry through Bioprinting: Personalization of Oral Tissues. J. Funct. Biomater. 2023, 14, 530. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14100530
Shopova D, Mihaylova A, Yaneva A, Bakova D. Advancing Dentistry through Bioprinting: Personalization of Oral Tissues. Journal of Functional Biomaterials. 2023; 14(10):530. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14100530
Chicago/Turabian StyleShopova, Dobromira, Anna Mihaylova, Antoniya Yaneva, and Desislava Bakova. 2023. "Advancing Dentistry through Bioprinting: Personalization of Oral Tissues" Journal of Functional Biomaterials 14, no. 10: 530. https://0-doi-org.brum.beds.ac.uk/10.3390/jfb14100530